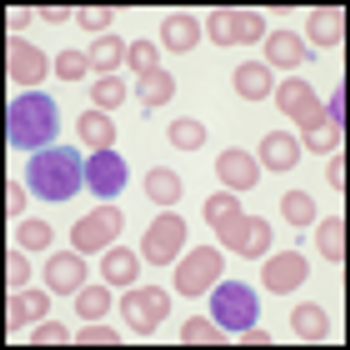
A phase III trial of the new drug idelalisib has been stopped early thanks to highly significant efficacy among patients with chronic lymphocytic leukemia (CLL).

Your AI-Trained Oncology Knowledge Connection!


A phase III trial of the new drug idelalisib has been stopped early thanks to highly significant efficacy among patients with chronic lymphocytic leukemia (CLL).

The FDA issued a safety alert and reports it is investigating a high rate of thrombosis and other cardiovascular events in patients taking ponatinib (Iclusig) for CML or Philadelphia chromosome-positive ALL.
Patients with primary central nervous system lymphoma had high response rates and good long-term disease control with a chemotherapy regimen followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in a new phase II trial.

The role of transplant in MCL is in clinical evolution. Up-front high-dose therapy and autologous stem cell transplant remains an attractive option for those with chemosensitive disease regardless of the induction regimen chosen, whereas this approach in the relapsed or refractory setting has not yielded long-term disease-free intervals.

This review will cover innovative therapeutic approaches in relapsed or refractory MCL, many of which have the potential to alter treatment paradigms toward the development of strategies that do not involve conventional chemotherapy agents.

The addition of the oral pan-deacetylase inhibitor panobinostat to bortezomib helped to elicit responses in about one-third of heavily pre-treated patients with multiple myeloma who were refractory of bortezomib, according to the phase II results of the PANORAMA 2 study.

A mutation of the PAX5 gene has been found to play an important role in inherited cases of B-cell precursor acute lymphoblastic leukemia (B-ALL). The mutation was isolated in two unrelated families with high propensity for B-ALL.

Gilead Sciences has submitted an NDA to the FDA for its new drug idelalisib, for the use in patients with indolent non-Hodgkin lymphoma that is refractory to rituximab and chemotherapy.

Pomalidomide, an immunomodulatory drug, combined with low-dose dexamethasone improved progression-free survival in patients with refractory or relapsed and refractory multiple myeloma compared with standard of care high-dose dexamethasone, according to a new study.

Evidence continues to mount that discontinuing imatinib treatment for chronic myeloid leukemia (CML) in the chronic phase is safe. A new phase II Dutch and Belgian study showed only about two-thirds of patients relapsed after discontinuing treatment with imatinib and cytarabine, and all patients remained sensitive to imatinib after relapse.

MZL comprises three different entities that require integration of clinical and pathologic features to make a diagnosis. Treatment is chosen and initiated on the basis of presentation, symptoms, and underlying subtype.

The number of recently approved agents and those under investigation is promising. However, there are currently no recommendations regarding the optimal timing for use of these agents, a reflection of the lack of data in this area and the need for prospective studies.

This is an exciting time in the treatment of PTCL, but with this opportunity comes responsibility. The challenge of how to optimize a plethora of promising new therapies for a small number of patients will drive therapeutic decision making for the foreseeable future.

To define the differences between the subtypes of myeloma patients, not only prospective collection of clinical and laboratory data are needed, but also cytogenetics and molecular profiling.

Myelomas that “lack” a monoclonal protein can be divided further into those that produce some protein and yet do not secrete it or whose serum concentration is so low that it cannot be measured, and those that truly produce no monoclonal protein at all.

In this article we briefly review the labeled indications for new agents for cutaneous and peripheral T-cell lymphoma, focus on data from the last 1 to 2 years, and on data from ongoing clinical trials, with the hope that in doing so we can help elucidate difficult treatment decisions.

Numerous small series of patients suggest that the prognosis for non-secretory myeloma patients is likely no worse than the prognosis for patients with traditional secretory myeloma, and in some settings may be superior.

A phase I study of dasatinib in children and adolescents with chronic myeloid leukemia (CML) and other malignancies determined dosing for further studies and suggested the drug is well tolerated and effective in these patients.

Patients with multiple myeloma who underwent autologous stem cell transplantation may have a continued response to the treatment even after the traditional disease assessment at 100 days. A new study indicates that this continued response maintained prognostic value and should be taken into account when considering post-transplant therapies.

Maintenance therapy with rituximab showed some improvement over observation, though it did not reach significance, among older patients with advanced follicular lymphoma who responded to a brief chemoimmunotherapy induction regimen followed by rituximab consolidation therapy.

A phase I study showed that azacitidine priming followed by standard chemotherapy could be an effective approach to resistance in patients with newly diagnosed diffuse large B-cell lymphoma (DLBCL).

Researchers in London have identified a number of new genetic variants that are linked to myeloma, and one specifically linked to a telomerase RNA component gene called TERC, that helps to control the aging process by acting as a cell’s internal clock.

The real promise of improving patient outcomes in IgD and IgE multiple myeloma lies in multi-drug combinations, next-generation agents, and immunotherapy.

The use of newer methods of disease assessment that focus on minimal residual disease may facilitate the long-term evaluation of IgD and IgE myeloma patients, even if the rare Ig subtype is not identified at diagnosis.

How often do we choose the “second best” treatment as initial therapy for our patients with cancer? The management of chronic myeloid leukemia should be no different.