Lymphoma
Latest News
Latest Videos

More News

Julie M. Vose, MD, MBA, discussed MZL research and future directions in the February Letter to Readers.

The TRANSCEND FL trial showed that liso-cel elicited an ORR of 97.1% and a complete response rate of 94.2% in patients with follicular lymphoma.

Researchers from the University of Wisconsin, Cornell, and a consortium of other institutions conducted a retrospective analysis on CD19-directed CAR T-cell therapy for R/R T-cell/histiocyte-rich LBCL.

Select patients may be eligible to continue treatment with tabelecleucel or ATA3219 in accordance with study protocols.

Tycel Phillips, MD, questioned how the regimen of acalabrutinib, bendamustine, and rituximab would compare with taking the drugs separately in mantle cell lymphoma.

“Some of the early data that came out of the [ECHO] trial that led to this approval does suggest that it may be beneficial in some high-risk patients,” Tycel Phillips, MD, said.

The CRL did not identify any deficiencies related to the manufacturing, efficacy, or safety outlined in the BLA, and no new clinical trials were requested.

Brentuximab vedotin, lenalidomide, and rituximab yielded a median OS of 13.8 months and a median PFS of 4.2 months in the phase 3 ECHELON-3 trial.

The FDA has approved acalabrutinib in previously untreated MCL based on results from the phase 3 ECHO trial.

Brad S. Kahl, MD, and Tycel Phillips, MD, discussed the use of acalabrutinib plus bendamustine and rituximab for patients with untreated MCL.

Here are 3 things you should know about immunotherapy in diffuse large B-cell lymphoma.

Results from the CLOVER WaM trial saw a clinical benefit rate of 98.2% in patients with Waldenström Macroglobulinemia treated with Iopofosine I 131.
Phase 3 data support tafasitamab plus lenalidomide/rituximab as a potential new standard of care in patients with relapsed/refractory follicular lymphoma.

Autologous transplant did not confer significantly improved overall survival regardless of induction intensity in a phase 3 trial.

Positive PFS and EFS Outcomes Were Elicited by Pirtobrutinib in CLL and SLL
Pirtobrutinib sustained BTK inhibition and improved progression-free survival in patients with CLL and SLL, data from the phase 3 BRUIN CLL-321 trial showed.
Glofitamab with polatuzumab vedotin benefited patients with high-grade B-cell lymphomas and who failed prior CAR T-cell therapy.

Phase 2 data support epcoritamab monotherapy as a promising chemotherapy-free option for older patients with newly diagnosed LBCL.
Findings from the phase 1/2 CaDAnCe-101 trial showed that promising ORRs in R/R WM and R/R CLL/SLL stemmed from BGB-16673 treatment.

For patients with CLL and SLL, sonrotoclax with zanubrutinib yielded high rates of overall response in the phase 1/1b BGB-11417-101 study.

Tisagenlecleucel shows high rates of MRD-negative status among patients with relapsed/refractory follicular lymphoma in the ELARA trial.

Almost a 100% complete response rate was noted with Zilovertamab Vedotin/R-CHP for patients with DLBCL.
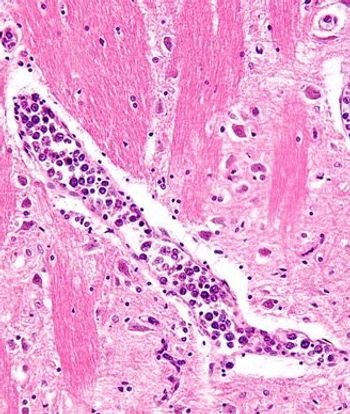
For patients with relapsed/refractory large B-cell lymphoma, PFS and OS data from CD-19-directed, 4-1BB CAR T-cell Liso-cel therapy were consistent with prior results.
For patients with relapsed or refractory diffuse large B-cell lymphoma in the US, tafasitamab elicited promising real-world efficacy outcomes.

For patients with relapsed/refractory diffuse large b-cell lymphoma, meaningful efficacy outcomes were shown with FS118.

The FDA is expected to decide on approving glofitamab in relapsed or refractory diffuse large B-cell lymphoma by July 20, 2025.