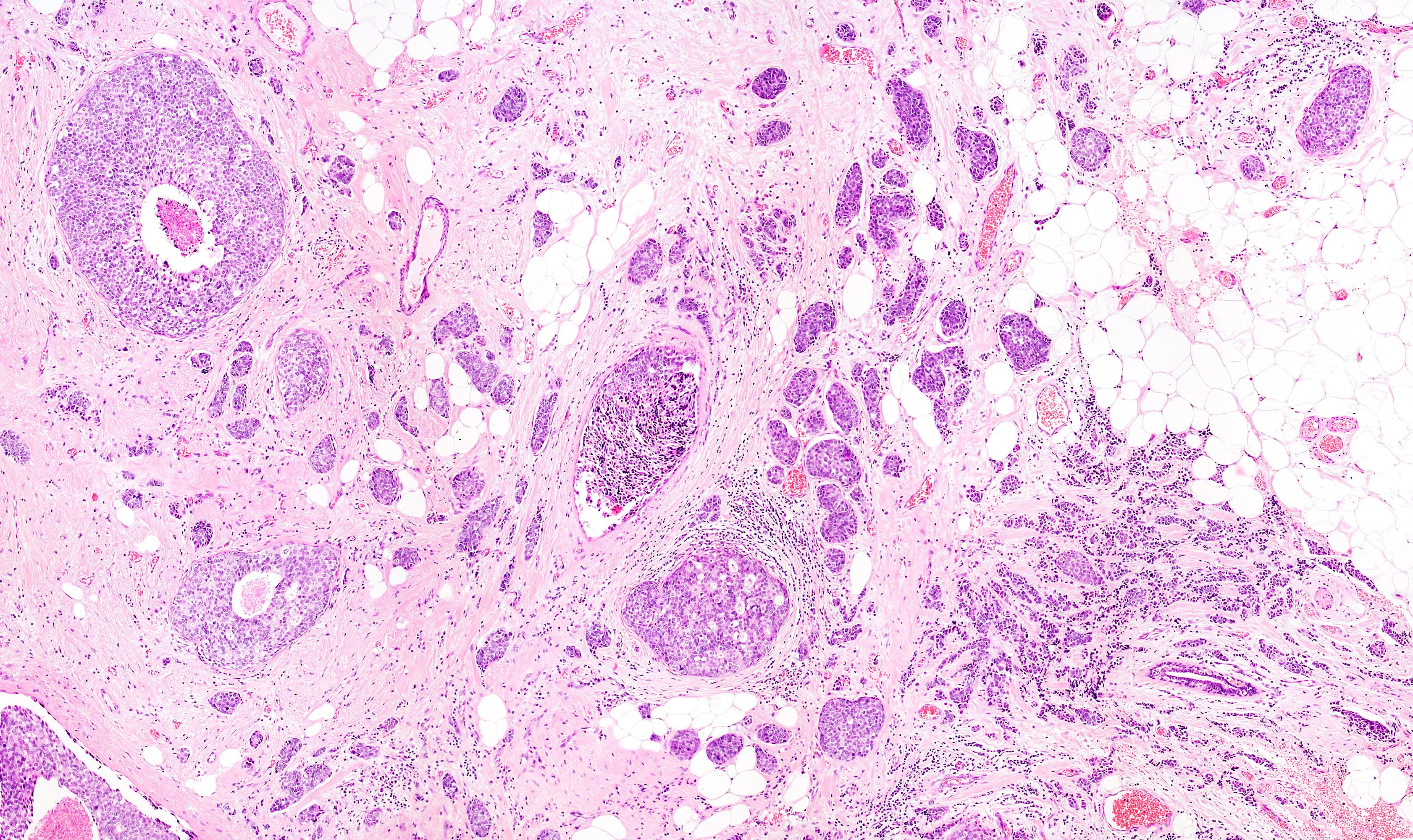
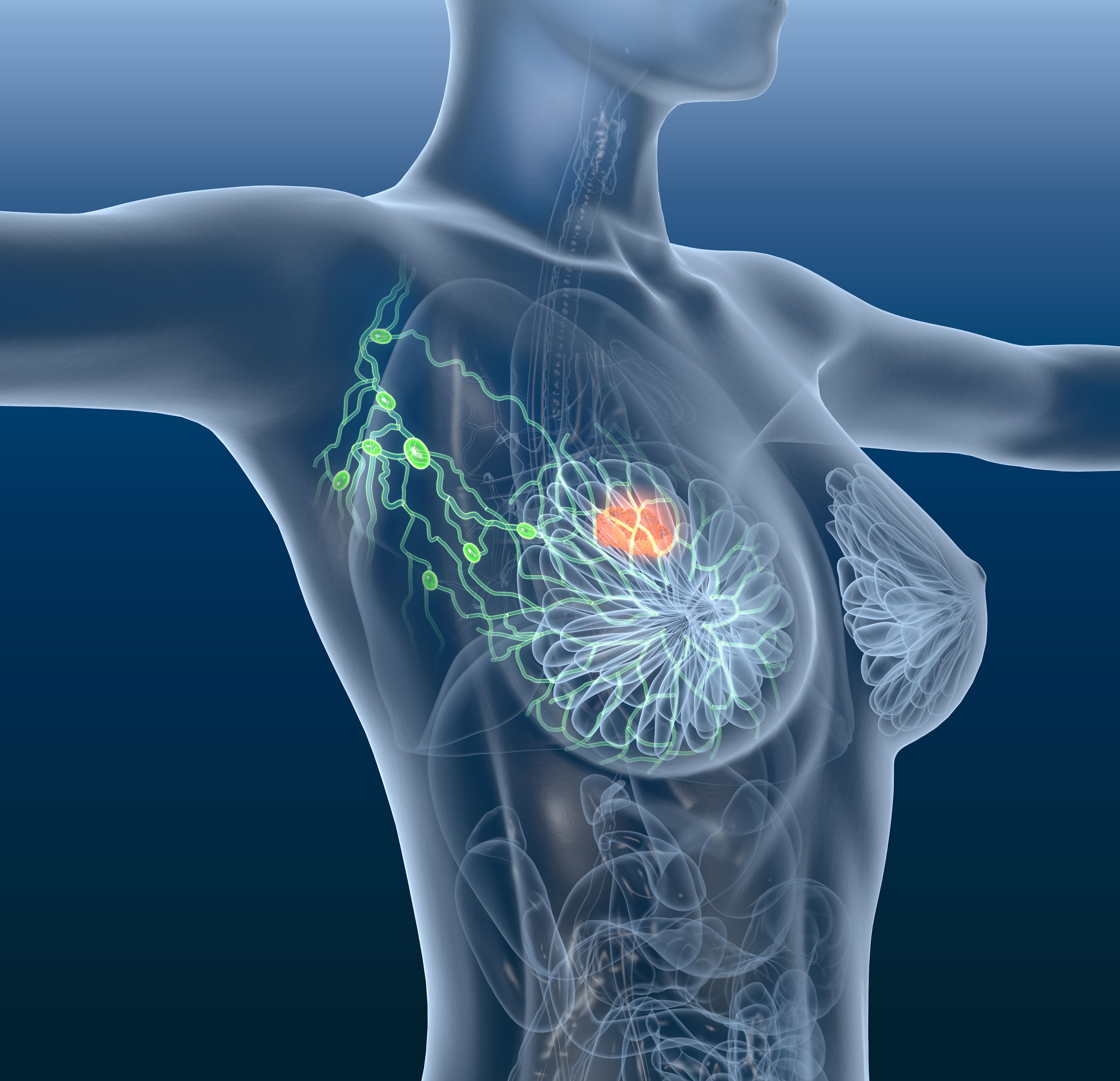
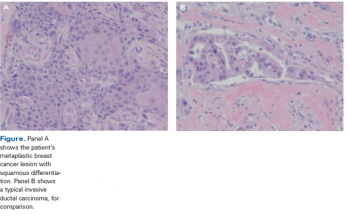
Final data from a phase 2 trial suggested that the administration of trilaciclib before chemotherapy comprised of gemcitabine and carboplatin resulted in a significant improvement in overall survival in previously treated patients with metastatic triple-negative breast cancer.