
In 2023, the FDA issued a warning on secondary primary malignancies after treatment with CAR T-cell therapy.

Your AI-Trained Oncology Knowledge Connection!


In 2023, the FDA issued a warning on secondary primary malignancies after treatment with CAR T-cell therapy.

Joshua Richter, MD, led an enthusiastic panel with his colleagues in the multiple myeloma space following the 2023 ASH Annual Meeting and Exposition.

Findings from the phase 3 CheckMate 649 trial support nivolumab plus chemotherapy as a standard frontline therapy for patients with gastric, gastroesophageal junction, and esophageal adenocarcinoma.

It may be applicable to administer CAR T-cell therapy to patients with large B-cell lymphoma in a community or outpatient setting.
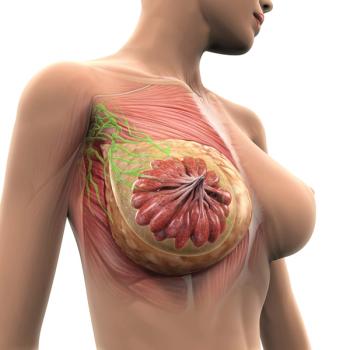
Targeted imaging and biopsy may accurately identify patients with breast cancer who do not have residual disease, according to Henry Kuerer, MD, PhD, FACS, CMQ.

Leonard G. Gomella, MD, FACS, discusses new clinical trials that are evaluating novel targeted therapy options for those with prostate cancer.

The use of a robot to aid in nipple-sparing surgery has been a controversial topic, although ongoing trials may show its potential.

Treatment with brentuximab vedotin, lenalidomide, and rituximab yielded a progression-free survival benefit in the phase 3 ECHELON-3 trial.

Patient-reported outcomes support the clinical benefits of ide-cel among patients with relapsed/refractory multiple myeloma.
Strategies must be framed for addressing racial and geographic disparities in access for CAR T-cell and bispecific antibody therapy, according to the study authors.

Treatment with simple hysterectomy reduces the incidence of urinary incontinence compared with radical hysterectomy in patients with low-risk cervical cancer.
Investigators plan to reinitiate enrollment of patients with non-Hodgkin lymphoma as part of the phase 1a/1b NX-2127-001 trial.

Jae Park, MD, discussed the use of CAR T-cell therapy across the hematologic malignancies space.

Primary care physicians cannot afford to dismiss younger people who present with symptoms of cancer in the face of increasing cancer incidence, according to Monique Gary, DO, MSc, FACS.

Findings from a study highlight that 7/8 mismatched unrelated donor posttransplant cyclophosphamide may be a suitable alternative treatment option for those with graft-vs-host disease.

Helena A. Yu, MD, and Sandip P. Patel, MD, reviewed the use of patritumab deruxtecan, which was assessed in the phase 2 HERTHENA-Lung01 trial.

The use of novel agents like tarlatamab may be “interesting” among patients with small cell lung cancer in the relapsed setting, says Gregory Peter Kalemkerian, MD.

Jun Gong, MD, and Daneng Li, MD, discussed the most impactful trials coming from 2024 ASCO GI.

Reshma Jagsi, MD, DPhil, highlights disparities in hypofractionation, toxicity, and cardiac doses in radiotherapy for Black and Asian patients with breast cancer.

Findings from the INSITE trial support the Medical Imaging Drugs Advisory Committee’s positive opinion on pegulicianine for breast cancer surgery.

Data from the phase 3 VIALE-A trial showed continued responses during long-term follow-up for patients with newly diagnosed acute myeloid leukemia treated with venetoclax/azacitidine.

Findings from a phase 2 trial support the premise that activating the androgen receptor may elicit antitumor effects in patients with androgen receptor–positive, estrogen receptor–positive, HER2-negative breast cancer.

Developers also announced that they completed enrollment of patients with non–small cell lung cancer in the phase 2 THIO-101 trial.

In a recent Hot Topics article, reimbursement rates for Medicare physicians are discussed, and how it will impact their practice.

Treatment with rusfertide may reduce the use of phlebotomy and limit debilitating disease-related symptoms in patients with polycythemia vera based on findings from the phase 2 REVIVE trial.
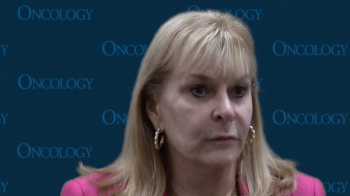
The FDA approval of zanubrutinib plus obinutuzumab showed a benefit to patients with relapsed/refractory follicular lymphoma, according to Julie Vose, MD, MBA.

Patients with relapsed/refractory follicular lymphoma can now receive zanubrutinib plus obinutuzumab based on the data from the phase 2 ROSEWOOD trial.

The phase 3 ALPINE and ASCEND studies were used in the MAIC analysis assessing zanubrutinib vs acalabrutinib in relapsed/refractory chronic lymphocytic leukemia.

Results from the phase 3 CHECKMATE-901 trial led to the approval of nivolumab plus cisplatin and gemcitabine for patients with unresectable or metastatic urothelial carcinoma.

The DREAMM-8 trial assessing belantamab mafodotin plus pomalidomide/dexamethasone met the primary end point for those with relapsed/refractory multiple myeloma.