
Patients with KRAS G12C-mutated non–small cell lung cancer who have brain metastases or intolerability of intravenous infusion may be more suitable to receive a small molecule inhibitor compared with chemotherapy, says Sandip P. Patel, MD.

Your AI-Trained Oncology Knowledge Connection!


Patients with KRAS G12C-mutated non–small cell lung cancer who have brain metastases or intolerability of intravenous infusion may be more suitable to receive a small molecule inhibitor compared with chemotherapy, says Sandip P. Patel, MD.

The FDA set a Prescription Drug User Fee Act date of June 15, 2024 for repotrectinib as a treatment for those with advanced or metastatic solid tumors harboring an NTRK gene fusion.

Results from a phase 2 trial support the safety and tolerability of VGT-309 in patients with suspected lung cancer who undergo standard-of-care surgical resection.

Rachel Rau, MD, gives her perspective on the use of asparaginase treatments for pediatric patients with acute lymphocytic leukemia.

The final overall survival analysis of trilaciclib plus chemotherapy in patients with metastatic triple-negative breast cancer as part of the PRESERVE 2 trial will take place in the third quarter of 2024.

Julie M. Vose, MD, MBA, discussed the use of collaboration in driving progress in mantle cell lymphoma research and bridging the gap between research and clinical care.

Patients with metastatic pancreatic ductal adenocarcinoma may now receive irinotecan liposome in combination with 5-fluorouracil/leucovorin and oxaliplatin in the first-line setting, which has been approved by the FDA.

Findings from a phase 1b trial support the tolerability and potential survival benefit of CAN-3110 as a treatment for patients with recurrent high-grade glioma.

Investigators of the AMPLIFY-201 trial hypothesized that ELI-002 2P may induce the expansion of functional tumor-directed KRAS-mutated specific T cells following tumor resection.

Treatment with sotorasib or adagrasib appears to be more tolerable among patients with KRAS G12C-mutated non–small cell lung cancer compared with docetaxel, according to Sandip Patel, MD.

Administering pegylated asparaginase continuously to pediatric patients with acute lymphoblastic leukemia appears to be safe without compromising the efficacy of treatment.

HEMO-CAR-T can now proceed with its evaluation as a treatment for those with acute myeloid leukemia as part of a phase 1 trial.

Findings from a phase 2 trial support the potential survival benefit of BXCL701 plus pembrolizumab in patients with small cell neuroendocrine prostate cancer.

Martin Dreyling, MD, spoke about the potential use of CAR T-cell therapy in earlier treatment stages for certain patient groups with mantle cell lymphoma.

Lisa Carter-Bawa PhD, MPH, APRN, ANP-C, FAAN, discusses how LungTalk, a health communication and decision support tool, may spread awareness and knowledge surrounding lung cancer screening.

The CROWN and ADURA trials were discussed and defended as part of a non–small cell lung cancer Face Off.

Elias Campo, MD, PhD, provides insight into biological subtyping and the tumor microenvironment relating to mantle cell lymphoma.

Data from the phase 3 MARIPOSA study support the Type II application for amivantamab plus lazertinib as a treatment for those with EGFR-mutated non–small cell lung cancer.

Vanderbilt University Medical Center and Winship Cancer Institute at Emory University Face Off on recent data in multiple myeloma and acute lymphoblastic leukemia.

Treatment with repotrectinib in patients with ROS1 fusion–positive non–small cell lung cancer results in mostly low-grade adverse effects in the phase 1/2 TRIDENT-1 trial.

Michael Wang, MD, gives an overview of the progress that has been made in mantle cell lymphoma research and where research should be focused on new treatment strategies and improved understanding of the disease.

Investigators discontinued the phase 3 ENHANCE-3 study after magrolimab plus azacitidine and venetoclax yielded an increased risk of death in those with acute myeloid leukemia.

The phase 3 ALLELE trial found that using tabelecleucel for patients with relapsed/refractory Epstein-Barr Virus–positive post-transplant lymphoproliferative disease improved efficacy outcomes, specifically for those with hematopoietic stem-cell transplant.

Resection can be safely performed in select patients with cT4 non–small cell lung cancer without compromising perioperative survival, according to Neel Chudgar, MD.

Treatment with second-line vepdegestrant in those with advanced breast cancer is currently under evaluation as part of the phase 3 VERITAC-2 trial.

In 2023, the Lymphoma Research Foundation hosted the Mantle Cell Lymphoma Scientific Consortium and Workshop to discuss treatment advancements in the field.
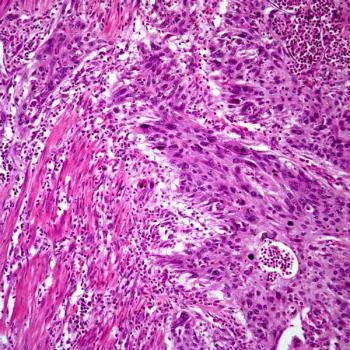
Interim findings from a phase 3 trial support adjuvant pembrolizumab as a new therapeutic option for those with muscle-invasive urothelial carcinoma at high risk of recurrence.

Both the FDA and European Medicines Agency applications were based on findings from the phase 3 CheckMate-77T trial in patients with resectable non–small cell lung cancer.

The FDA sets a Prescription Drug User Fee Action date of June 21, 2024 for blinatumomab as a treatment for those with CD19-positive B-cell precursor acute lymphoblastic leukemia.

Results from the phase 3 DREAMM-7 trial highlighted an efficacy benefit with belantamab mafodotin plus bortezomib and dexamethasone in patients with relapsed/refractory multiple myeloma.