
Oncodermatology is critical in managing adverse effects from cancer treatments, specifically targeted therapies and immunotherapies.

Your AI-Trained Oncology Knowledge Connection!


Oncodermatology is critical in managing adverse effects from cancer treatments, specifically targeted therapies and immunotherapies.

Ankit Bharat, MBBS, a thoracic surgeon, discussed potential treatment-emergent adverse effects or complications as well as strategies for managing them.

Experts in gynecologic cancer, genitourinary malignancies, and other disciplines highlight noteworthy clinical data slated for presentation at ASCO 2025.


Among patients with rectal cancer who underwent total mesorectal excision following SCRT plus camrelizumab and chemotherapy, the 3-year OS rate was 93.3%.
!["[I]nduction [etoposide and carboplatin] followed by a combination of camrelizumab, apatinib and [etoposide and carboplatin], and subsequent maintenance camrelizumab plus apatinib, showed a tolerable safety and promising antitumor activity, suggesting its potential as a first-line therapy option for patients with ES-SCLC," according to the study authors.](https://cdn.sanity.io/images/0vv8moc6/cancernetwork/b1587a6320f32b9e3dab51f09b50bb01961a2d68-1200x946.jpg?w=350&fit=crop&auto=format)
The safety profile of camrelizumab plus apatinib and chemotherapy in a phase 1 study aligns with prior reports of each agent.

The Jack & Sheryl Morris Cancer Center offers “state-of-the-art” surgical suites and advanced radiation technology, says Andrew M. Evens, DO, MBA, MSc.

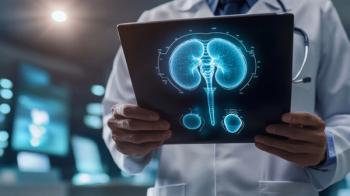
Prostate-specific membrane antigen PET imaging has completely altered the landscape of prostate cancer detection and management. Read as several experts discuss the trajectory and growth of these agents.
!["Given the superior efficacy of [chemoimmunotherapy], it holds promise as a first-line neoadjuvant therapy for MIBC, providing greater benefits to patients," according to the study authors.](https://cdn.sanity.io/images/0vv8moc6/cancernetwork/481e483e3d7f2b45988df26b6d358730cc4c7acb-1200x800.jpg?w=350&fit=crop&auto=format)
Real-world data may support chemotherapy plus immune checkpoint blockade as a promising frontline neoadjuvant therapy for muscle-invasive bladder cancer.

Thoracic surgeon Ankit Bharat, MBBS, discussed surgical strategies among patients with advanced lung cancer diagnoses based on staging.

The European Commission is expected to approve the belantamab mafodotin combinations for this multiple myeloma population in the third quarter of 2025.


The EMA’s CHMP has adopted a positive opinion for mirdametinib in patients with inoperable plexiform neurofibromas of neurofibromatosis type 1.

In a CancerNetwork® YouTube video, Cornelia Tischmacher, a mother of twins from Germany, outlined her receipt of double lung transplantation.

From breast cancer to head and neck tumors, the 2025 ASCO Annual Meeting may feature a wide range of practice-changing data across cancer care.

The positive CHMP opinion is based on results from the phase 1b/2 FELIX trial evaluating obe-cel in relapsed/refractory B-cell ALL.

Data from the INCITE-ES study show 100% technical success with pulsed electric field energy delivery before surgical resection.

AI-assisted training dramatically reduced HER2-null overscoring and improved sensitivity in HER2-low and HER2-ultralow breast cancer cases.


The phase 3 IMforte trial evaluating lurbinectedin plus atezolizumab is the first to show PFS and OS improvement with first-line maintenance for ES-SCLC.
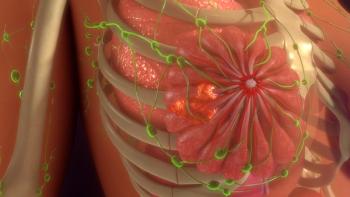
Final OS data from INAVO120 demonstrate, for the first time, a significant OS improvement with a PI3K-targeted agent in this breast cancer population.

There was a 7% lower incidence of obesity-related cancer with GLP-1 receptor agonist use.
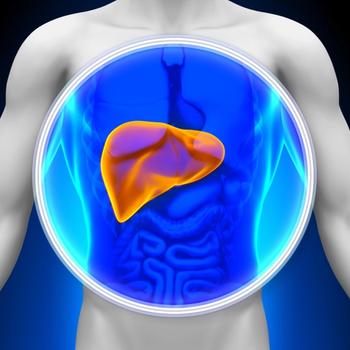
Survival and safety data from the phase 3 CheckMate 9DW trial showed that nivolumab plus ipilimumab was superior to lenvatinib or sorafenib in advanced HCC.

Tafasitamab is the first CD19 antibody approved in China for the treatment of patients with relapsed or refractory diffuse large B-cell lymphoma.

Kit Yu Lu, MD, discussed the implications of the TROPiCS-02 and DESTINY-Breast06 trial data for sequencing ADCs in HR+/HER2– breast cancer.

Decipher Prostate, Oncotype DX, and Prolaris are 3 gene expression testing options for patients with prostate cancers. Read as experts discuss them.

Eight votes were cast against the favorability of talazoparib and enzalutamide in the first-line setting for patients with metastatic castration-resistant prostate cancer.


Given an unmet need for efficacious therapies against ALK-positive NSCLC, experts discuss optimal ALK TKI sequencing for patients with this disease.