
There are certain markers that can point to a greater risk of pancreatic cancer like BRCA1/2, and diabetes, according to Brian M. Wolpin, MD.

Your AI-Trained Oncology Knowledge Connection!


There are certain markers that can point to a greater risk of pancreatic cancer like BRCA1/2, and diabetes, according to Brian M. Wolpin, MD.

Survivors of cancer may experience an increased risk of having organ, cardiac, or lung disease following prior anti-cancer therapy.

The pancreatic cancer field may not be far from seeing the use of non-invasive blood tests in pancreatic cancer, said Ajay Goel, PhD, AGAF.
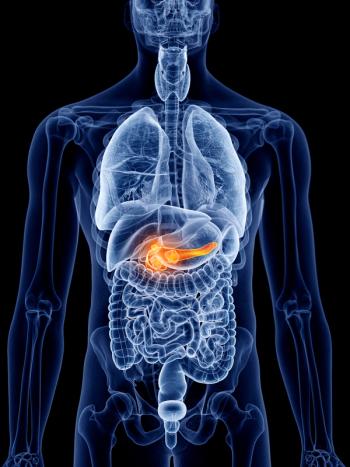
Tumor treating fields with chemotherapy improved overall survival for patients with unresectable, locally advanced pancreatic adenocarcinoma.
Identifying inefficiencies in the health care system, such as excessive regulations, highlights its impact on the oncology-based audience.

CancerNetwork co-hosts Kristie L. Kahl and Andrew Svonavec highlight abstracts to look out for surrounding the multidisciplinary approach at the upcoming ASH Annual Meeting in San Diego, with some additional tidbits to round out the main event.

Only a few groups of patients get screened for pancreatic cancer, those with a genetic risk or pancreatic cysts among them, which can increase lethality for unidentified populations.

The development of RAS-directed vaccines may help decrease the likelihood of disease recurrence in patients undergoing treatment for pancreatic cancer.
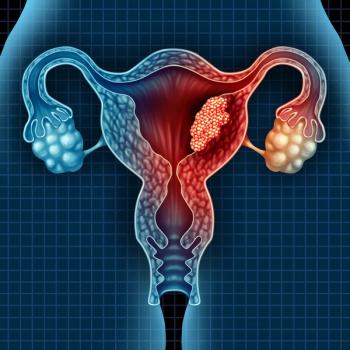
Risk factors and biomarker testing in conjunction with cancer staging may better identify ideal treatment for endometrial cancer than cancer staging alone.

The European Commission has approved ribociclib plus an aromatase inhibitor for patients with early breast cancer.

Ablative technology may generate an immune response that can be enhanced via injected immunotherapy in patients with solid tumors.

A nurse practitioner discussed risk factors, diagnostic challenges, and treatment planning in patients with pancreatic cancers.

The key to the future of pancreatic cancer treatment is immunotherapy, according to Gregory L. Beatty, MD, PhD.
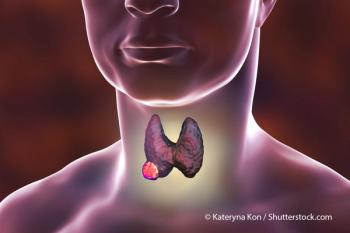
For patients with head and neck squamous cell carcinoma and contraindications to cisplatin, durvalumab yielded worse PFS vs cetuximab.

Bolstering the effectiveness of T cells and the further implementation of AI can both yield positive results in the future of pancreatic cancer treatment.
Shubham Pant, MD, MBBS, highlights how pan-RAS inhibitors, RAS-directed vaccines, and biomarker testing can improve outcomes in pancreatic cancer.

Immunohistochemistry presents a solution to the obstacles that the different identified cellular neighborhoods in pancreatic tumors create.
Treatment with vorasidenib also confers better seizure control than placebo in the phase 3 INDIGO study.

Researchers are trying to figure out how to create immune memory with immunotherapy in patients with pancreatic cancer.
ASTRO assembled a task force to create a guideline for the administration of palliative external beam radiation therapy for patients with symptomatic bone metastases.

Medical use of AI increases every day, and in the future, will be exponentially greater and many forms of treatment will be improved, according to Russell C. Langan, MD, FACS, FSSO.

Performing ablation and injecting tumor sites with immunotherapy may be “synergistic”, according to Jason R. Williams, MD, DABR.

The European Commission has approved tislelizumab plus chemotherapy as treatment for patients with esophageal and gastric or GEJ cancer.
Despite gaps in biomarker testing accessibility, the lung cancer survival rate has improved by 26% over the last 5 years.

Shubham Pant, MD, MBBS, highlights an “exciting time” in the treatment of patients with RAS-mutated pancreatic cancer.

Greater direct access to academic oncologists may help address challenges associated with a lack of CAR T education in the community setting.

A computational linguistics model designed to locate pancreatic cysts that started to locate pancreatic cancer has the potential to lead to more efficient treatment.

Tiragolumab with atezolizumab, when compared with placebo with atezolizumab, failed to reach its overall survival end point in patients with NSCLC.

For patients with KRAS-mutated colorectal cancer, onvansertib plus FOLFIRI/bevacizumab was effective in those who had not received prior bevacizumab.

Brett L. Ecker, MD, discusses the importance of multidisciplinary collaboration in improving patient outcomes in neuroendocrine tumors.