Prostate Cancer
Latest News
Latest Videos

CME Content
More News
Switching to a patient scenario of metastatic hormone-sensitive prostate cancer, experts discuss which therapeutic approach they would undertake.
A brief discussion on the role of PSMA-targeted therapy’s role in managing patients with nonmetastatic castration-resistant prostate cancer.

Patients with metastatic castration-resistant prostate cancer who had a high genomic loss of heterozygosity may respond better to treatment with talazoparib.

Genetic determinants of PSA proved to robustly predict prostate cancer diagnoses and improve the detection of aggressive disease; however, larger and more diverse studies are still required.

Steven Finkelstein, MD, DABR, FACRO, and Louis J. Mazzarelli, MD, react to the manuscript titled, “A Prospective Head-to-Head Comparison of 18F-Fluciclovine With 68Ga-PSMA-11 in Biochemical Recurrence of Prostate Cancer in PET/CT,” by Birgit Pernthaler and colleagues.

Neeraj Agarwal, MD, and Simon Chowdhury, MD, review final results from the TITAN study of apalutamide in patients with mCSPC and discuss the clinical implications of the findings.

Phillip S. Low, PhD, pioneered development of 177Lu-PSMA-617 for patients with metastatic castration-resistant prostate cancer and spoke about its recent approval.

Patients with prostate cancer who received surgery had the highest rate of treatment-related regret followed by radiotherapy and active surveillance.

Based on data from the phase 3 VISION trial, 177Lu-PSMA-617 may now be used to treat patients who were previously treated with androgen receptor pathway inhibitors plus taxane-based chemotherapy for metastatic prostate specific membrane antigen–positive castration-resistant prostate cancer.
Axel Merseburger, MD, PhD, spoke about implementing the treatment combination of enzalutamide plus docetaxel/prednisone for progressive castration-resistant prostate cancer from the PRESIDE trial into the real-world.
Dr. Bobby Liaw discusses importance of setting expectations regarding common side effects and challenges patients might experiences with treatment.

A prostate cancer expert shares rationale for his preferences and choices while sequencing therapies in prostate cancer.
At a recent conference, Robert Dreicer, MD, MS, MACP, FASCO, offered his advice regarding the use of androgen deprivation therapy in patients with metastatic castration-sensitive prostate cancer.

Dr Liaw discusses ways to measure response to treatment and what recent data regarding PSA kinetics has shown.

Dr Bobby Liaw talks about relationship between treatment duration, dosing and efficacy.
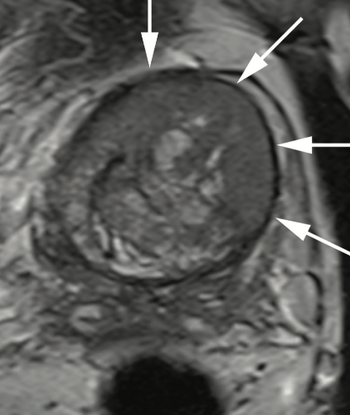
Dr. Judd W. Moul, MD, and colleagues present the case of a man, aged 73 years, with a prostate-specific antigen level of 110 ng/mL after 4 negative prostate biopsies and 4 negative prostate MRIs.

Axel Merseburger, MD, PhD, looked back on 2022 ASCO GU and the new data presented for the treatment of prostate cancer.

In this program Atish D. Choudhury, MD, PhD, discusses recent updates on long-term safety and efficacy data in patients with metastatic castration-sensitive prostate cancer and the importance of patient communication and quality of life while on treatment.

Dr. Atish Choudhury discusses safety features to keep in mind when choosing androgen receptor pathway inhibitors.

A prostate cancer expert shares long-term data with androgen receptor pathway inhibitors in mCSPC.

Dr. Liaw shares the role disease burden and other factors play in treatment selection.

Dr. Choudhury discusses treatment considerations and treatment options for mCSPC.

Applications for darolutamide were submitted to the FDA and European Medicines Agency for the treatment of patients with non-metastatic hormone-sensitive prostate cancer.
The addition of prostate radiation therapy to androgen deprivation therapy appears to be cost effective in patients with low-volume metastatic hormone-sensitive prostate cancer.

Eleni Efstathiou, MD, PhD, spoke about how results of the phase 3 MAGNITUDE trial, which examined the combination of niraparib with abiraterone acetate and prednisone, have the ability to change the standard of care in patients with metastatic castration-resistant prostate cancer.