Researchers have uncovered two mutations in thyroid tumors that make the tumor either very sensitive to treatment with everolimus, or confers resistance.

Your AI-Trained Oncology Knowledge Connection!


Researchers have uncovered two mutations in thyroid tumors that make the tumor either very sensitive to treatment with everolimus, or confers resistance.

Results of a new study show that cancer rates among children and adolescents are stable, but rates of certain cancers such as renal carcinomas are increasing.

Josh Kremer, MD, vice president of clinical development at Eisai, Inc, discusses results of the phase III SELECT trial, which studied lenvatinib in radioiodine-refractory differentiated thyroid cancer.

An analysis of thyroid cancer diagnosis and incidence in the United States suggests that there is an epidemic of diagnosis rather than an epidemic of thyroid cancer per se.

The FDA has approved sorafenib (Nexavar) for the treatment of metastatic differentiated thyroid cancer.
Data presented at ASCO from the phase III DECISION trial showed treatment with the TKI sorafenib (Nexavar) delayed disease progression by 5 months in patients with metastatic differentiated thyroid cancer that had progressed on radioactive iodine.

The US Food and Drug Administration (FDA) has approved cabozantinib (Cometriq) for the treatment of metastatic medullary thyroid cancer, a rare type of thyroid cancer that makes up about 4% of all cases.
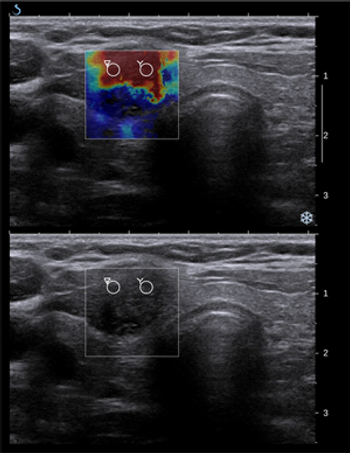
A new study shows that obese patients are more likely to have advanced papillary thyroid cancer and for the cancer to be of an aggressive subtype.

In rare diseases, the “magic” lies in close collaboration between individual investigators at academic institutions, the pharmaceutical industry, and funding institutions such as the National Cancer Institute.

Like Burnison and Lim, we conclude conveying our sense of optimism that progress is being made-and that important clinical questions are being asked related to the care of patients afflicted with ATC. We believe, however, that in the final analysis, important progress will remain highly dependant upon collaborations conducted across specialties, across institutions, and across nations.

In this article, we endeavor to clarify the role of radiation therapy and chemotherapy in the treatment of ATC; we note important contributions of the historical literature, and we review more contemporary strategies adopted by several renowned institutions.
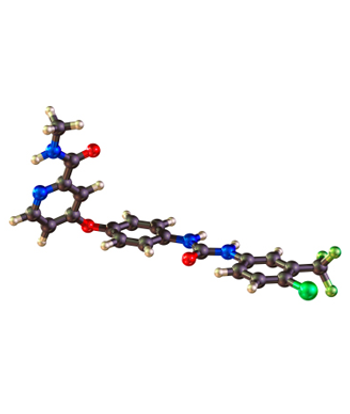
AstraZeneca today announced that the orphan drug vandetanib is now available to U.S. patients for the treatment of medullary thyroid cancer that cannot be removed by surgery or that has spread to other parts of the body.
The US Food and Drug Administration approved the first treatment for a rare type of thyroid cancer on Friday April 6, 2011.

In response to the radiation spreading from the No. 3 reactor of the Fukushima Daiichi nuclear complex in Japan, sales of potassium iodide have soared. Some have claimed, however, that the short-term benefits of the drug are a myth, as the radioiodine is less likely to be inhaled, and more likely to be ingested in the future, often from drinking milk produced from cows grazing in areas affected by radiation fallout.

Pazopanib represents a therapeutic option for patients with advanced differentiated thyroid cancers. A phase II trial conducted at the Mayo Clinic in Rochester, Minn., included 39 patients with metastatic, rapidly progressive, radioiodine-refractory differentiated thyroid cancers.

Current treatment methods may offer little more than overtreatment, while follow up should not automatically mean expensive and unnecessary tests.

TORONTO-Two large, population-based studies with over 35 years of data revealed some of the factors that have influenced the incidence of thyroid cancer. Two key findings: Canadian men and people living in rural areas generally present with more advanced disease and the incidence of anaplastic thyroid cancer is waning. The studies were presented at the 2009 World Congress on Thyroid Cancer.
About 30,000 new cases of thyroid cancer are diagnosed annually in the United States.[1] The incidence among men has risen more dramatically than any other malignancy in recent years (2.4% annual increase).[2] Thyroid cancers arise from one of two cell types, namely follicular and parafollicular cells.

In this helpful review, the authors catalog a number of the novel molecular agents now being examined for treatment of radioiodine-resistant, metastatic differentiated thyroid cancer. They also call for increased systematic study of outcomes through recruitment of patients into large-scale trials.

The paper by Higgins et al published in this issue highlights the important advances that have been made in the treatment of advanced thyroid cancer over the past few years. Patients with iodine-refractory metastatic thyroid cancer have suffered badly due to the reputation of thyroid cancer as being a “good” cancer to have.

Over the past 3 decades, the incidence rate of testicular seminoma has continually risen, and the majority of cases have been clinical stage I.[1] Nevertheless, the overall survival for all testicular cancers has improved significantly (P < .05) over the same period, from 83% to 96%.[2]

Lawrentschuk and Fleshner accurately depict the difficulty in choosing among observation, prophylactic radiation, and adjuvant chemotherapy for clinical stage I testicular seminoma. The physican has competing priorities of avoiding unnecessary treatment while minimizing the overall burden of both therapy and surveillance testing. The patient has to contend with defined risks that exist with any of the three options.

Patients with papillary thyroid cancer typically undergo a triad of consecutive initial treatments, comprising surgery, radioiodine therapy, and thyroid hormone suppression of serum thyrotropin (thyroid-stimulating hormone, or TSH).

Differentiated thyroid cancer, the most common endocrine malignancy, can touch the lives of young and old individuals. It is generally associated with a normal lifespan whether it is completely eradicated or held in check with judicious medical interventions.

There is uniform agreement that initial treatment of high-risk thyroid cancer should include total thyroidectomy, compartmental neck dissection of clinically involved cervical lymph nodes, and radioactive iodine (RAI) remnant ablation.