
Results of the PREDATOR-MRD trial found that daratumumab could help predict MRD response for patients with multiple myeloma.

Your AI-Trained Oncology Knowledge Connection!


Results of the PREDATOR-MRD trial found that daratumumab could help predict MRD response for patients with multiple myeloma.


Compared with standard therapy, dasatinib did not improve efficacy for patients with core-binding factor AML.

More than 80% of patients who were screened for cervical cancer and provided with a self-collection kit did so by utilizing the kit.

Biomarker analyses indicate the predictive potential of a 15-protein signature related to the efficacy of serplulimab plus chemotherapy in ES-SCLC.

Patients treated with nivolumab in the phase 3 CheckMate 577 trial were less likely to experience progression-related treatment discontinuation vs placebo.

JNJ’4496 at 75 M CAR T cells elicited an ORR of 100% in patients with relapsed or refractory large B-cell lymphoma who received 1 prior line of treatment.
Results of a post hoc analysis found spleen and symptom response rates to be comparable with ESAs or danazol in combination with ruxolitinib for patients with myelofibrosis who have anemia.

Data from APPULSE-PNH may support oral iptacopan as a potentially practice-changing option in patients with paroxysmal nocturnal hemoglobinuria.

Treatment with cyclosporin/cyclophosphamide following allogeneic stem cell transplant had improved efficacy vs cyclosporin/methotrexate in patients with high-risk hematologic cancers.
Results from the phase 3 POD1UM-303/InterAACT 2 trial demonstrated improved PFS and OS in patients with locally recurrent or metastatic SCAC.
The FDA decision follows phase 2 CANFOUR trial data showing a 2-year survival rate of 35% in IL1RAP-high metastatic pancreatic ductal adenocarcinoma.

Patients with R/R DLBCL given polatuzumab vedotin, rituximab, gemcitabine, and oxaliplatin had an OS of 19.5 months.

Considering which non–muscle-invasive bladder cancer cases may be cured by surgery alone may help mitigate overtreatment in this patient group.

Data from the phase 3 KEYNOTE-689 trial support the approval of the pembrolizumab-based regimen in this locally advanced HNSCC population.


The VIALE-A trial showed high activity with revumenib plus azacitidine and venetoclax for patients with NPM1m/KMT2Ar AML.

Event-free survival benefit was observed among BCG-naive patients with carcinoma in situ undergoing treatment with sasanlimab plus BCG.

Tetiana Skrypets, MD, highlighted that older adults with PTCL may be treated as younger adults if they pass the geriatric assessment.

Data from the phase 3 ENVISION trial support the FDA approval of the mitomycin solution for patients with recurrent, low-grade, intermediate-risk NMIBC.

The developer plans to share top-line results for RAD101 in various solid tumors from the supporting phase 2b trial in the second half of 2025.
In patients 39 years or younger, a statistically significant association between colorectal cancer diagnosis and endometriosis was not observed.

Phase 3 data demonstrate the potential utility of BRAF/MEK inhibition in the adjuvant setting for patients with stage IIB/IIC BRAF V600–mutant melanoma.

Zanubrutinib tablets were found to have the same efficacy and safety as capsules based on 2 single-dose, open-label randomized phase 1 studies.
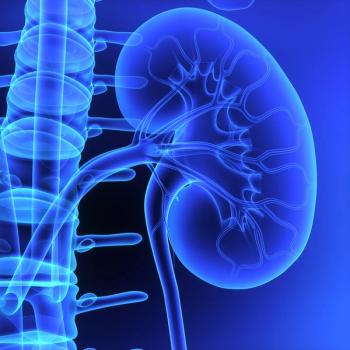
There appeared to be no extra benefit with the addition of nivolumab to tivozanib among patients included in the phase 3 TiNivo-2 trial.

The safety profile of iopofosine I 131 in the phase 1b CLOVER-2 trial appears consistent with prior reports of the agent.

Data from the ELEVATE trial may support elacestrant as an endocrine therapy backbone in ER-positive, HER2-negative metastatic breast cancer.

Supporting results for the approval of taletrectinib in ROS1+ NSCLC come from the TRUST-I and TRUST-II trials.

Counseling received prior to colorectal surgery using 3-dimensional–printed anatomic models reduced mean anxiety scores vs conventional counseling.

Data from the ENVISION trial may support UGN-102 as a well-tolerated, efficacious treatment in non–muscle-invasive bladder cancer.