
Sacituzumab govitecan showed anti-tumor activity in patients with pretreated metastatic or locally recurrent head and neck squamous cell carcinoma.

Your AI-Trained Oncology Knowledge Connection!


Sacituzumab govitecan showed anti-tumor activity in patients with pretreated metastatic or locally recurrent head and neck squamous cell carcinoma.

Patients with recurrent ovarian cancer did not see an improvement in efficacy when given atezolizumab plus chemotherapy and niraparib.
The phase 3 KEYNOTE-756 trial yielded an increased pathological complete response for those with high-risk, early-stage, estrogen receptor–positive, HER2-negative breast cancer receiving pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab plus endocrine therapy.

Results from the phase 1/2 STARTRK-NG and phase 2 TAPISTRY trials led to the approval of entrectinib for patients who are 1 month or older with solid tumors.
Pembrolizumab combination yielded an improved progression-free survival benefit vs placebo in patients with newly diagnosed, previously untreated, high-risk locally advanced cervical cancer.
Results from the phase 2 PERLA trial showed an improved objective response and overall survival in treatment with dostarlimab plus chemotherapy in patients with treatment-naïve, nonsquamous non–small cell lung cancer.
Pembrolizumab appears to improve overall survival vs placebo when added to trastuzumab and chemotherapy among patients with metastatic HER2-positive gastric or gastroesophageal junction cancer.

Resolving disparities within the blood cancer space is a matter of “access to excellent healthcare,” according to Usama Gergis, MD, MBA.
Findings from the ENZA-p study provide evidence for enhanced anti-cancer effects with 177Lu-PSMA-617 plus enzalutamide in patients with metastatic castration-resistant prostate cancer.
Event-free survival benefits with pembrolizumab plus chemotherapy in the phase 3 KEYNOTE-522 trial appear to be consistent in triple-negative breast cancer across subgroups based on nodal status, tumor size, and PD-L1 status.

Data from the phase 2 KRYSTAL-7 study support a phase 3 trial assessing concurrent adagrasib plus pembrolizumab compared with pembrolizumab alone in treatment-naïve KRAS G12C-mutated non–small cell lung cancer.
Findings from a secondary data analysis suggest a need for further research to determine risk factors that place younger patients who are Puerto Rican at a high risk of endometrial cancer.

Tarlatamab may represent a new immunotherapeutic approach for patients with small cell lung cancer, according to the investigators of the phase 2 DeLLphi-301 trial.
Adding durvalumab to standard-of-care chemotherapy appears to improve downstaging in patients with resectable gastric cancer and gastroesophageal junction cancer in the phase 3 MATTERHORN study.

Results from the phase 3 FLAMES trial show an improved progression-free survival benefit with senaparib monotherapy vs placebo across all pre-specified patient subgroups with newly diagnosed, advanced ovarian cancer.

Five-year data from the monarchE trial support adding adjuvant abemaciclib to endocrine therapy for patients with hormone receptor–positive, HER2-negative breast cancer.

UT Southwestern and Mayo Clinic debated over current treatment options in chronic myeloid leukemia and multiple myeloma.
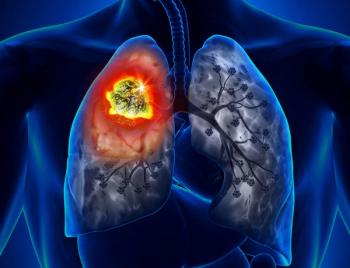
Data from a phase 1a/1b trial support additional research on dual TIGIT/PD-L1 inhibition in patients with advanced solid tumors.

Fred Hutch and City of Hope "Face Off" regarding presentations focusing on multiple myeloma and chronic myeloid leukemia.
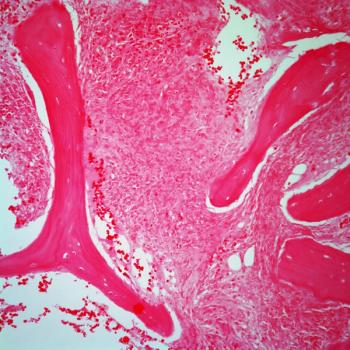
Data from the phase 3 ECHELON-1 trial support the European Commission’s approval of brentuximab vedotin plus chemotherapy as a treatment for those with CD30-positive stage III Hodgkin lymphoma.

Developers plan to assess onvansertib plus standard of care as a frontline treatment for patients with metastatic pancreatic ductal adenocarcinoma in an investigator-initiated trial.
A real-world study found most patients with lower-risk myelodysplastic syndrome do not appear to require dose escalations of luspatercept.
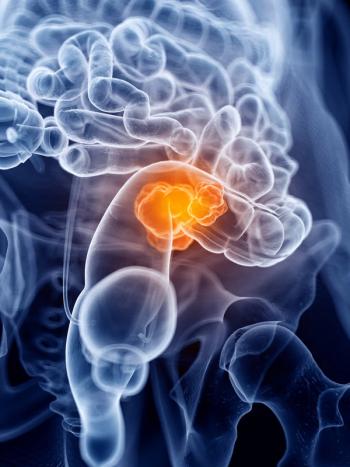
Approval of the CRCdx RAS Mutation Detection Kit may improve access to panitumumab for the treatment of patients with colorectal cancer.

Tanios S. Bekaii-Saab, MD, gave an overview of colorectal cancer along with current standard of care, and potential treatment options.

Hypofractionated radiotherapy yields less financial toxicity than conventionally fractionated radiotherapy in patients with breast cancer who have undergone reconstruction following mastectomy.
Rates of treatment discontinuation appear to be higher in patients receiving nivolumab plus ipilimumab for cancer than those receiving nivolumab monotherapy.
Investigators discussed the potential link between secondary pure red cell aplasia and subcutaneous daratumumab/hyaluronidase formulation for multiple myeloma.
The FDA companion diagnostic designation for the FoundationOne CDx and FoundationOne Liquid CDx may improve access to treatment with encorafenib plus binimetinib for those with BRAF V600E-mutated non–small cell lung cancer.
Findings from a meta-analysis highlight the effect of varying censorship criteria for the definition of surrogate end points in localized prostate cancer randomized clinical trials.

Those with resectable non–small cell lung cancer can now receive neoadjuvant treatment with pembrolizumab and chemotherapy followed by adjuvant pembrolizumab after approval by the FDA.