
Currently, it is not known whether cisgender and transgender patients with cancer respond similarly to standard-of-care therapies due to challenges collecting gender identity and sexual orientation data in clinical cancer research.

Your AI-Trained Oncology Knowledge Connection!


Currently, it is not known whether cisgender and transgender patients with cancer respond similarly to standard-of-care therapies due to challenges collecting gender identity and sexual orientation data in clinical cancer research.

The Partnership Director at GRYT Health opened up about their collaboration with Bristol Myers Squibb to address disparities in clinical trials.

Don Dizon, MD, emphasizes the importance of policies ensuring higher levels of gay and transgender participation in clinical cancer trials.
Phase 3 data suggest that concurrent treatment with ibrutinib and venetoclax yielded promising efficacy for patients with relapsed/refractory mantle cell lymphoma.
Attention needs to be paid to the psychosocial needs of patients, especially those with poor health-related quality of life, who have little support, or are single mothers.

For patients with HER2-positive metastatic breast cancer, CD8-positive, cytotoxic tumor infiltrating lymphocytes may be predictive of outcomes after treatment with trastuzumab.
In an interview with CancerNetwork®, Andrew Hendifar, MD discusses a case series analysis focused on real-world outcomes with targeted and standard therapies in a subset of patients with pancreatic cancer and RAF alterations.

Use of a multidisciplinary geriatric assessment-driven intervention resulted in a reduction in grade 3 or higher chemotherapy-related toxic events.

Despite 5-year survival increasing nationally for patients with lung cancer, patients of color continue to experience poor survival.

Patients with intermediate-risk and high-risk renal cell carcinoma had a statistically significant improvement in disease-free survival after being treated with pembrolizumab, which was approved by the FDA.
Phase 3 data indicated that the combination of nivolumab and ipilimumab followed by treatment with dabrafenib and trametinib, if necessary, resulted in greater overall survival at 2 years for patients with treatment-naïve BRAF-mutant metastatic melanoma.

Patients with newly diagnosed glioblastoma saw improved progression-free survival when treated with tumor treating fields, pembrolizumab, and temozolomide versus historical control data.

Patients with advanced cancers who were ineligible for trials were more likely to receive frontline immune checkpoint inhibitor monotherapy than patients with the same advanced cancers who were eligible for trials.
CancerNetwork®sat down with John Burke, MD, to talk about the topline results in the KEYNOTE-564 trial, which examined the use of pembrolizumab in a population of patients with high-risk renal cell carcinoma.
Patients who developed brain metastases from renal cell carcinoma experienced promising intracranial activity after being treated with cabozantinib.
Despite the importance of incorporating palliative care into strategies for metastatic kidney cancer, little data exists in the space, highlighting a need for further research.

Patients with melanoma, head and neck squamous cell carcinoma, and cervical cancer who had not previously received immunotherapy and were treated with lifileucel plus pembrolizumab experienced promising overall response rates compared favorably with historical data on pembrolizumab monotherapy.

CancerNetwork®sat down with John Burke, MD, to discuss why treatment with pembrolizumab post-surgery may be a promising option for patients with high-risk renal cell carcinoma.

Patients with relapsed/refractory adult B-cell acute lymphoblastic leukemia who were treated with AUTO1 experienced durable responses.
Patients with hormone receptor–positive breast cancer in 3 separate subgroups showed an overall survival benefit when treated with eftilagimod alpha plus paclitaxel compared with the placebo.
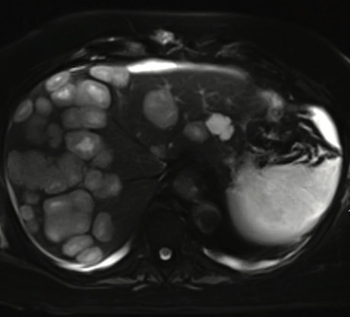
Ghulam Gous, MD, and colleagues report a case of glioblastoma with extensive liver metastases along with a review of previous reports of liver metastasis from glioblastomas and the possible mechanisms of metastasis.

CancerNetwork® sat down with Hossein Borghaei, DO, MS, to talk about the findings of the DESTINY-Lung01 study that were presented at 2021 ESMO.
In an interview with ONCOLOGY®, David Hughes PharmD, BCOP, and Jessica Freydman, PharmD, offers a comprehensive review of real-world treatment considerations of tepotinib as therapy for patients with metastatic non–small cell lung cancer that have MET exon14 skipping mutations.

“I’ve done a lot of work [not only with] clinical trials, but also understanding the disease, the prognostic factors, and the management of adverse effects.”

Adult patients with polycythemia vera may now be treated with ropeginterferon alpha-2b, the only interferon available for this population.
PSA testing rates for prostate cancer increased among men after the US Preventive Services Task Force released updated guidance in 2017 that reversed the recommendations from previous guidance in 2012.
Patients with melanoma who have asymptomatic brain metastases had long-lasting responses after being treated with first-line nivolumab and ipilimumab.

Cell therapy omidubicel, designed for patients with hematologic malignancies who are undergoing allogeneic stem cell transplant, will be submitted to the FDA for approval in 2022.
A select group of patients with metastatic renal cell carcinoma may benefit from cytoreductive nephrectomy followed by active surveillance as an alternative option to systemic therapy.
Patients with microsatellite instability-high/mismatch repair deficient metastatic colorectal cancer treated with first-line nivolumab plus low-dose ipilimumab experienced a durable clinical benefit.