
Patients saw an improvement in overall survival when given fam-trastuzumab deruxtecan-nxki to treat HER2-positive gastric cancer or gastroesophageal junction adenocarcinoma.

Your AI-Trained Oncology Knowledge Connection!


Patients saw an improvement in overall survival when given fam-trastuzumab deruxtecan-nxki to treat HER2-positive gastric cancer or gastroesophageal junction adenocarcinoma.
Patients with unresectable hepatocellular carcinoma received an overall survival benefit following first-line treatment with durvalumab (Imfinzi) and tremelimumab.

When compared with placebo and chemotherapy, durvalumab plus gemcitabine and cisplatin was superior in terms of overall survival in the treatment of advanced biliary tract cancer.
CYNK-101 plus standard frontline chemotherapy, trastuzumab, and pembrolizumab has received a fast track designation from the FDA for patients with advanced HER2-positive gastric or gastroesophageal junction adenocarcinoma.

Mehmet Sitki Copur, MD, gives his perspective on liquid biopsies and how they can be utilized in gastrointestinal cancer.

The International Society of Gastrointestinal Oncology (ISGIO), hosted by Physicians’ Education Resource, LLC (PER®) presented the latest advances in the field of gastrointestinal cancer research.
Patients with cholangiocarcinoma may derive benefit from treatment with TT-00420, which was recently given a fast track designation by the FDA.
Patients with locally advanced or metastatic biliary tract cancer who received either ramucirumab or merestinib in addition to standard first-line cisplatin and gemcitabine did not experience further survival benefit, although safety remained tolerable.

Patients with advanced biliary tract cancer received a clinically meaningful and statistically significant overall survival benefit after being treated with durvalumab and chemotherapy.
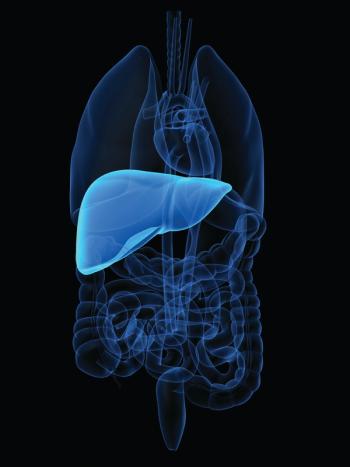
Patients with unresectable hepatocellular carcinoma appeared to benefit from treatment with durvalumab and tremelimumab, according to findings from the phase 3 HIMALAYA trial.

Treatment with nivolumab/ipilimumab and nivolumab/chemotherapy has demonstrated efficacy in unresectable advanced, recurrent, or metastatic esophageal squamous cell carcinoma, with results from the CheckMate-648 trial leading to the acceptance of the regimens’ applications by the FDA.
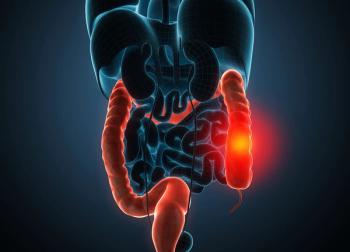
A final overall survival analysis found improved results and a tolerable safety profile for patients with advanced IDH1-mutated cholangiocarcinoma who received ivosidenib vs placebo.
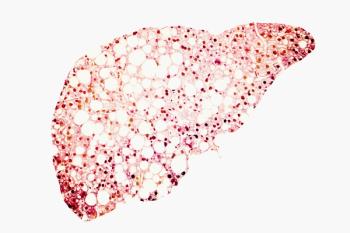
Patients with advanced hepatocellular carcinoma who received prior treatment with sorafenib appear to benefit from treatment with pembrolizumab and best supportive care.

Takayuki Yoshino, MD, PhD provides perspective on key issues surrounding therapy selection in gastrointestinal cancers.
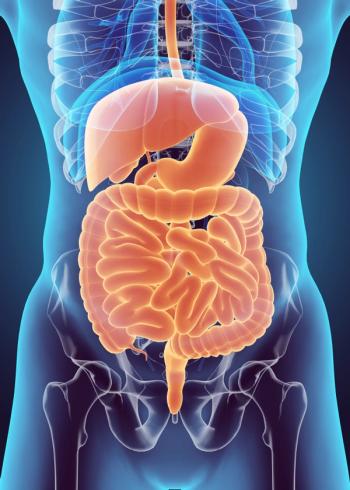
Pembrolizumab plus chemotherapy compared with the placebo plus chemotherapy demonstrated an improvement in overall survival and progression-free survival for patients with esophageal squamous cell carcinoma.
Data presented at 2021 ESMO show that nivolumab plus chemotherapy improves survival outcomes in certain patients with frontline gastrointestinal cancers, but not nivolumab plus ipilimumab.
Results from the phase 3 JUPITER06 trial demonstrated positive outcomes with toripalimab plus platinum-based chemotherapy in patients with treatment-naïve advanced or metastatic esophageal squamous cell carcinoma.

Updated results of DESTINY-Gastric02 trial show antitumor response with trastuzumab deruxtecan in patients with in certain patients with HER2-positive gastric/gastroesophageal junction.

Results of 2 studies presented at 2021 ESMO demonstrate superior efficacy of sintilimab plus chemotherapy versus chemotherapy alone in certain gastrointestinal cancers.
Patients with HER2-positive, metastatic biliary tract cancer experienced promising responses and a tolerable safety profile following treatment with pertuzumab plus trastuzumab.

The NovoTTF-200T System was given a breakthrough designation by the FDA as treatment strategy that may work in conjunction with bevacizumab and atezolizumab to treat patients with advanced liver cancer.
Data from patients with gastric or gastroesophageal junction cancers who were treated with nivolumab showed that effects in the genomic pathways of the gut microbiome may be significantly associated with survival.

Oncomine Dx Target Test is now approved by the FDA as a companion diagnostic for patients with IDH1-mutant cholangiocarcinoma who may be candidates for treatment with ivosidenib.

The phase ORIENT-16 trial indicated that the addition of sintilimab to chemotherapy significantly improved of overall survival compared with placebo.
Oncologists can now use the International Esodata Study Group’s risk predictor model to identify patients with esophageal cancer who might be at a very high-risk of death following an esophagectomy.