
This year's American Society of Clinical Oncology meeting featured a highly select lineup of sessions dealing with hematologic malignancies.

Your AI-Trained Oncology Knowledge Connection!

Zoledronic acid plus standard therapy offers anticancer effect in multiple myeloma

This year's American Society of Clinical Oncology meeting featured a highly select lineup of sessions dealing with hematologic malignancies.

Standard therapy options are limited for patients with refractory chronic lymphocytic leukemia, with or without lymphadenopathy, and the results are generally poor. Ofatumumab (Arzerra), a novel human CD20 monoclonal antibody, could be the answer for improving outcomes in this patient population. Lead investigator William G. Wierda, MD, PhD, will share the final analysis of the results from this international study at ASH 2010.

Anaplastic large cell lymphoma (ALCL) is a biologic and clinically heterogenous subtype of T-cell lymphoma. Clinically, ALCL may present as localized (primary) cutaneous disease or widespread systemic disease. These two forms of ALCL are distinct entities with different clinical and biologic features. Both types share similar histology, however, with cohesive sheets of large lymphoid cells expressing the Ki-1 (CD30) molecule. Primary cutaneous ALCL (C-ALCL) is part of the spectrum of CD30+ lymphoproliferative diseases of the skin including lymphomatoid papulosis. Using conservative measures, 5-year disease-free survival rates are>90%. The systemic ALCL type is an aggressive lymphoma that may secondarily involve the skin, in addition to other extranodal sites. Further, systemic ALCL may be divided based on the expression of anaplastic lymphoma kinase (ALK) protein, which is activated most frequently through the nonrandom t(2;5) chromosome translocation, causing the fusion of the nucleophosmin (NPM) gene located at 5q35 to 2p23 encoding the receptor tyrosine kinase ALK. Systemic ALK+ ALCLs have improved prognosis compared with ALK-negative ALCL, although both subtypes warrant treatment with polychemotherapy. Allogeneic and, to a lesser extent, autologous stem cell transplantation play a role in relapsed disease, while the benefit of upfront transplant is not clearly defined. Treatment options for relapsed patients include agents such as pralatrexate (Folotyn) and vinblastine. In addition, a multitude of novel therapeutics are being studied, including anti-CD30 antibodies, histone deacetylase inhibitors, immunomodulatory drugs, proteasome inhibitors, and inhibitors of ALK and its downstream signaling pathways. Continued clinical trial involvement by oncologists and patients is imperative to improve the outcomes for this malignancy.

Parents and their children need to understand that advancing science does not always go hand-in-hand with a direct benefit to the patients.
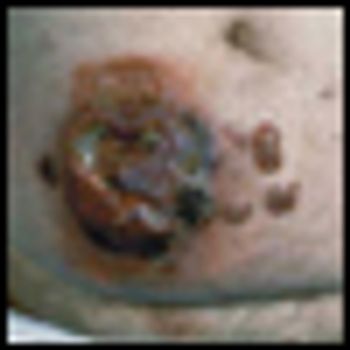
Mycosis fungoides (MF), the most common cutaneous T-cell lymphoma, is a low-grade cutaneous lymphoma characterized by skin-homing CD4+ T cells. It is notable for highly symptomatic progressive skin lesions, including patches, plaques, tumors, and erytheroderma, and has a poorer prognosis at later stages. Diagnosis remains difficult owing to MF’s nonspecific skin presentation and identification of the optimal treatment strategy is challenging given the paucity of controlled trials and numerous and emerging treatment options. Management includes topical therapy with the addition of systemic therapy for patients with later-stage disease including tumors; erythroderma; and nodal, visceral, or blood involvement. Topical therapies include mechlorethamine (nitrogen mustard), carmustine (BCNU), steroids, bexarotene gel (Targretin Gel), psoralen plus ultraviolet A (PUVA), ultraviolet B (UVB), and either localized or total skin electron radiotherapy. Systemic therapies include interferon, retinoids, oral bexarotene (Targretin), denileukin diftitox (Ontak), vorinostat (Zolinza), extracorporeal photochemotherapy (photopheresis), and cytotoxic chemotherapy. Herein, we outline clinically relevant aspects of MF, including clinical presentation, pathology, diagnosis, and staging. We describe in detail existing and emerging therapeutics and offer specific recommendations for management of each stage of MF.

The article entitled “Diagnosis and Management of Mycosis Fungoides” by Shira Galper, Benjamin Smith, and Lynn Wilson is an excellent contemporary summary of the workup and management of mycosis fungoides (MF) and its leukemia variant, Sézary syndrome (SS). In their discussion of the diagnosis and staging of MF and SS, the authors include a discussion of proposed revisions by the International Society for Cutaneous Lymphoma and the European Organisation for the Research and Treatment of Cancer (ISCL/EORTC) which seek to identify prognostic subgroups. In addition, there is a complete overview of the various treatment options for management of MF and SS. This treatment overview closely parallels the 2010 National Comprehensive Cancer Network (NCCN) Practice Guidelines for MF and SS.

Galper et al. should be commended for their concise and useful review of the diagnosis and management of mycosis fungoides (MF). It is notable that all of the authors are radiation oncologists. While the reader may expect a radiation oncologist’s perspective on the management of mycosis fungoides, their review goes beyond highlighting the various radiation techniques used in the treatment of MF. It highlights the major diagnostic dilemmas when evaluating patients with skin lesions that eventually are diagnosed as MF or its leukemic counterpart, Sézary syndrome (SS). It also stresses the importance of a multidisciplinary approach in diagnosing and caring for MF patients involving dermatology, dermatopathology, radiation oncology, and hematology/oncology, and provides a concise review of the treatment options in the MF and SS armamentarium. Navigating these options requires a good understanding of the natural history of the disease, the side effects of treatment, the expected response rates of treatment, the median time to response, the patient’s comorbid conditions, and goals of care.
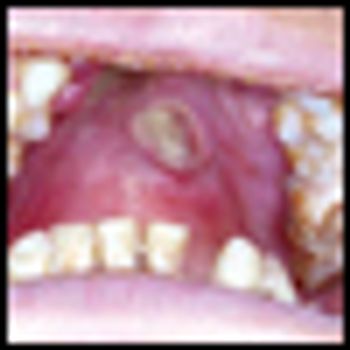
With better disease definition, staging, and monitoring, treatment of extranodal NK/T-cell lymphoma is becoming more rational. A large proportion of patients with localized nasal disease may enjoy prolonged disease-free survival. On the other hand, early HSCT or novel therapy may be recommended for aggressive extranasal disease.

Dr. Bennett and colleagues have provided a thorough and balanced history of the rise and fall of erythropoietin-stimulating agents (ESAs) in cancer-associated anemia. Their review encourages us to think about the lessons learned from this history-lessons about medical progress, the importance of clinical research in guiding clinical practice, and the role of the US Food and Drug Administration (FDA) in protecting patients

The perception and reality of the clinical value of erythropoiesis-stimulating agents (ESAs) in cancer supportive care have undergone a dramatic transformation since their initial use in 1990. The perception of ESA value in patients has evolved from panacea to miscreant over a 2-decade period of laboratory research, clinical trial data, and postmarketing experience. Meanwhile, the real clinical benefits of ESAs have changed very little from those described in the joint American Society of Clinical Oncology/American Society of Hematology guidelines originally published in 2002.[1] Even then, the value of initiation of ESAs was clear only in patients with hemoglobin values < 10 g/dL; quality-of-life measures produced inconsistent and, therefore, clinically inapplicable, results; and ESA use was shown to reduce the proportion of patients requiring red blood cell (RBC) transfusions by approximately 20%. The reality of ESA use that came to light following approval was increased mortality rates in certain populations, higher tumor progression and cancer recurrence rates, and more frequent and severe serious adverse effects including thromboembolism, stroke, and cardiovascular events.

Multiple myeloma remains the second most common hematologic malignancy in the United States, after non-Hodgkin lymphoma.

Anemia is a widely prevalent complication among cancer patients. At the time of diagnosis, 30% to 40% of patients with non-Hodgkin lymphoma or Hodgkin lymphoma and up to 70% of patients with multiple myeloma are anemic; rates are higher among persons with myelodysplastic syndromes. Among patients with solid cancers or lymphomas, up to half develop anemia following chemotherapy. For almost 2 decades, erythropoiesis-stimulating agents (ESAs) were the primary treatment for cancer-related anemia. However, reassessments of benefits and risks of ESAs for cancer-associated anemia have occurred internationally. We reviewed guidelines and notifications from regulatory agencies and manufacturers, reimbursement policies, and utilization for ESAs in the cancer and chronic kidney disease settings within the United States, Europe, and Canada. In 2008 the US Food and Drug Administration (FDA) restricted ESAs from cancer patients seeking cure. Reimbursement is limited to hemoglobin levels < 10 g/dL. In the United States, ESA usage increased 340% between 2001 and 2006, and decreased 60% since 2007. The European Medicines Agency (EMEA) recommended that ESA benefits do not outweigh risks. In Europe between 2001 and 2006, ESA use increased 51%; since 2006, use decreased by 10%. In 2009, Canadian manufacturers recommended usage based on patient preferences. In Canada in 2007, approximately 20% of anemic cancer patients received ESAs, a 20% increase since 2004. In contrast to Europe, where ESA use has increased over time, reassessments of ESA-associated safety concerns in the United States have resulted in marked decrements in ESA use among cancer patients.

Hematopoietic malignancies account for 6% to 8% of new cancers diagnosed annually. In the year 2009, an estimated 44,790 new cases of leukemia were diagnosed, and 21,870 deaths were attributable to leukemias of all types. The total age-adjusted incidence of leukemia, including both acute and chronic forms, is 9.6 per 100,000 population; the incidence of acute lymphoblastic leukemia (ALL) is 1.5 per 100,000 and of acute myelogenous leukemia (AML) is 2.7 per 100,000 population.

The incidence rates of non-Hodgkin lymphoma (NHL) in the United States have almost doubled between 1970 and 1990, representing one of the largest increases of any cancer. Although the overall incidence rates of NHL began to stabilize in the late 1990s, the temporal trends varied by histologic subtype. Some of this increase may be artifactual, resulting from improved diagnostic techniques and access to medical care, or directly related to the development of NHL in 25- to 54-year-old men with human immunodeficiency virus (HIV) infection. However, additional factors must be responsible for this unexpected increase in frequency of NHL that has been observed throughout the United States.

In 2009 approximately 8,510 new cases of Hodgkin lymphoma (HL) will be diagnosed in the United States. Over the past 4 decades, advances in radiation therapy and the advent of combination chemotherapy have tripled the cure rate of patients with HL. In 2009, more than 80% of all newly diagnosed patients can expect a normal, disease-free life span.

Induction therapy with higher daily doses of daunorubicin improved complete response and boosted overall survival in younger patients with acute myelogenous leukemia, according to results of the phase III ECOG E1900 trial.

Patients with lower-risk myelodysplastic syndromes and anemia can derive long-term benefits from erythropoietin and myeloid growth factor hormones, according to a study in Blood.

Peripheral T-cell lymphomas (PTCLs) are uncommonly encountered malignancies in the United States, and hepatosplenic T-cell lymphoma (HSTCL), subcutaneous panniculitis-like T-cell lymphoma (SPTCL), and enteropathy-type T-cell lymphoma (ETTCL) are rare subtypes of PTCLs that often present with primarily extranodal disease. Despite the fact that these tumors have distinct clinical and pathologic features, they are often diagnosed after significant delay. The combination of delay in diagnosis with ineffective therapies has resulted in a poor prognosis in most cases. Techniques that identify T-cell receptor gene rearrangements and flow cytometry that can identify characteristic immunophenotypes have guided our understanding of the underlying cell of origin of these rare PTCLs. As knowledge regarding the biology of these lymphomas increases alongside the development of newer therapeutics with novel mechanisms, clinicians must accordingly improve their familiarity with the clinical settings in which these rare malignancies arise as well as the pathologic features that make them unique

Despite the significant progress that has occurred in recent decades in the treatment of many advanced malignancies, skeletal morbidity remains a major problem for patients affected by cancers that metastasize to or grow primarily within bone.[1] Thus as patients with a variety of malignancies survive longer, therapies to limit cancer-associated as well as treatment-associated skeletal complications have become increasingly important for the provision of optimal patient care.

Research presented at the 51st Annual ASH Meeting explored optimal induction therapies for managing multiple myeloma and a potential first-line therapy for patients with non-Hodgkin lymphoma (NHL).

Adult T-cell leukemia/lymphoma (ATL) is defined as a histologically or cytologically proven peripheral T-cell malignancy associated with a retrovirus, human T-cell lymphotropic virus type I (HTLV-1).[1] Southwestern Japan is the district with the highest prevalence of HTLV-1 infection and the highest incidence of ATL in the world. A high prevalence of HTLV-1 infection is also found in the Caribbean islands, tropical Africa, South America, and northern Oceania.

Eltrombopag (Promacta) is the first orally absorbed, small-molecule, thrombopoietin receptor (TPO-R) agonist, approved (on November 20, 2008) by the US Food and Drug Administration (FDA) for the treatment of chronic immune thrombocytopenia (ITP) in patients who have relapsed following treatment with corticosteroids, immunoglobulins, and/or splenectomy.

The purpose of this review is to familiarize oncologists with the clinical and pathologic features of this relatively rare disease spectrum. This should enable appropriate clinical management and reassurance of patients concerned about their prognosis.

One of the greatest challenges facing the physician caring for patients with chronic lymphocytic leukemia (CLL) is the heterogeneity of this disease. Over the past decade, there have been major advances in understanding the pathophysiology of CLL, and in the identification of biomarkers that are helpful to predict the clinical course for individual patients. Over the same period, the available therapeutic options have developed dramatically, exemplified by the introduction of combination therapy with purine analogs and monoclonal antibodies, resulting in significant opportunities to induce complete remission (CR) in CLL patients.

Chronic lymphocytic leukemia (CLL) is a heterogeneous disease with an extremely variable course. Survival after diagnosis can range from months to decades. As the pathogenesis of the disease is increasingly understood, we begin to unfold the molecular patterns that define the different prognostic subgroups and to develop strategies to predict the clinical course.