The American Society of Hematology released guidelines that “take providers through the conversations they have with newly diagnosed patients, almost in real-time.”

Your AI-Trained Oncology Knowledge Connection!



The American Society of Hematology released guidelines that “take providers through the conversations they have with newly diagnosed patients, almost in real-time.”

The FDA cleared the clonoSEQ assay to detect and monitor minimal residual disease in blood or bone marrow from patients with chronic lymphocytic leukemia.
The MD Anderson Cancer Center expert discussed the future of acute myeloid leukemia treatment, and how the field is evolving rapidly.

Biosight Ltd announced that the FDA granted fast track designation to aspacytarabine for the treatment of adult patients aged 75 years or older with acute myeloid leukemia.
The MD Anderson Cancer Center expert discussed next steps in the evaluation of ivosenidib plus venetoclax, with or without azacytidine, in patients with IDH1-mutated acute myeloid leukemia.
The CLL expert spoke about the implications of this study for patients and how patients can apply this knowledge to their own course of treatment.
The MD Anderson Cancer Center expert spoke about the efficacy of ivosenidib plus venetoclax, with or without azacytidine, in patients with IDH1-mutated acute myeloid leukemia.

FACT-Leu was found to be a suitable outcome measure for patients not eligible for intensive therapy in acute myeloid leukemia clinical trials.
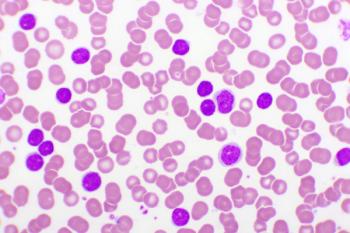
Researchers suggested that the use of the oral agent over a prolonged period of time should now be considered part of the overall treatment plan in acute promyelocytic leukemia.
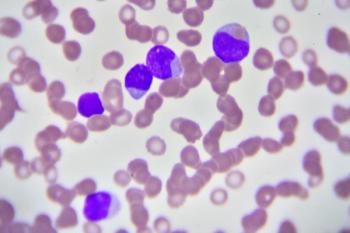
The chronic lymphocytic leukemia expert spoke about the possible use of venetoclax plus dose-adjusted R-EPOCH in various hematologic patient populations.
At the 2020 ASCO Virtual Program, Courtney DiNardo, MD, presented on a study of the combination therapy consisting of ivosenidib plus venetoclax with or without azacytidine patients with IDH1-mutated acute myeloid leukemia.
A multicenter phase 2 study of patients with chronic lymphocytic leukemia who developed Richter’s syndrome, presented at the 2020 ASCO Virtual Scientific Program, assessed the use of this treatment combination.

The FDA extended the indication of gemtuzumab ozogamicin (Mylotarg) for newly diagnosed CD33-positive acute myeloid leukemia to include pediatric patients who are 1 month and older.
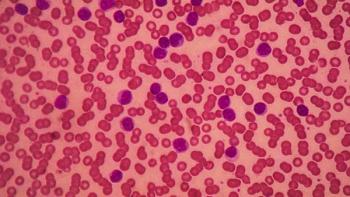
Both the phase 2 ACE-CL-001 trial and the pivotal phase 3 ASCEND trial showed the long-term efficacy and tolerability of acalabrutinib in patients with chronic lymphocytic leukemia.
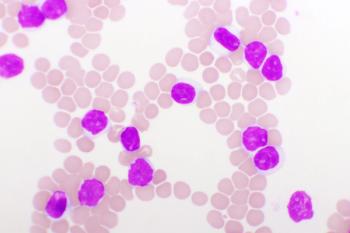
The study evaluated venetoclax plus dose-adjusted R-EPOCH in patients with chronic lymphocytic leukemia who developed Richter’s syndrome.
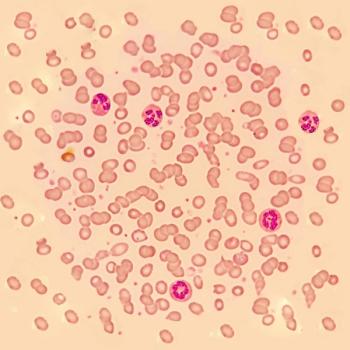
A phase II/III study of JZP-458 in patients with acute lymphoblastic leukemia/lymphoblastic lymphoma who are hypersensitive to E. coli-derived asparaginases was announced at the 2020 ASCO Virtual Scientific Program.

Trends in favor of better clinical outcomes were observed for those on-trial in this retrospective matched cohort study of patients with cancer who were treated on a phase III clinical trial compared with those who received standard therapy and/or were off trial.

Stephen Schuster, MD, talked about the benefits of conducting routine visits remotely during the COVID-19 pandemic, allowing doctors to see and treat more patients safely and efficiently.
Stephen Schuster, MD, discussed how they are prioritizing patients with more aggressive lymphomas, with emphasis on tumor volume and serum LDH levels during the COVID-19 pandemic.

The lymphoma and myeloma expert indicated that one of the key ways to address these disparities in lymphoma and myeloma is to improve minority and rural accrual in clinical trials.
Stephen Schuster, MD, explained how Penn Medicine is utilizing at-home treatments, which will continue after the pandemic, to maximize safety and reduce hospital traffic during the COVID-19 pandemic.
A study found that dexrazoxane, a cardioprotective drug, preserved cardiac function without compromising overall survival and event-free survival for pediatric patients with acute myeloid leukemia.
Stephen Schuster, MD, of Penn Medicine discussed testing for COVID-19, telemedicine and how they are adjusting their treatment of patients with aggressive lymphomas during the pandemic.
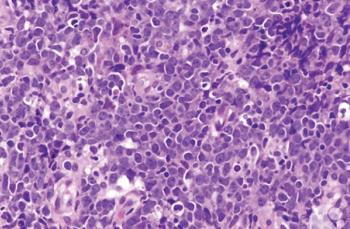
Test your diagnostic knowledge with this month's Image IQ.
Researchers found that a high dose of CAR T-19 may be more effective than a low dose at inducing a complete response without excessive toxicity.