Multiple Myeloma
Latest News
Latest Videos

CME Content
More News

Prognostic awareness impacted patients quality of life through psychosocial distress and symptoms burden.
Nina Shah, MD, discusses some of the important phase 3 trials to emerge from the previous year in multiple myeloma.

Nina Shah, MD, touched on novel immunotherapies and other emerging therapies for patients with multiple myeloma.

Nina Shah, MD, details the process to choose optimal therapeutic options for patients with relapsed/refractory multiple myeloma.

Nina Shah, MD, on BCMA-directed treatments for patients with multiple myeloma.

A panel of experts discuss strategies for optimizing first-line therapy for transplant-ineligible patients with newly diagnosed multiple myeloma.
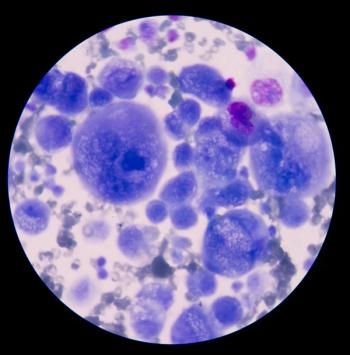
Experts in the multiple myeloma field discuss which front-line therapy is best for patients who are treatment ineligible.

Nina Shah, MD, discussed how adverse events impact treatment decision-making for patients with multiple myeloma.

Nina Shah, MD, discusses how to properly navigate treatment plans for patients with relapsed/refractory multiple myeloma.

CancerNetwork® spoke with Rafael Fonseca, MD, about the rationale for a simulation using real-world data sets to compare the utility of daratumumab-containing regimens as either frontline or second-line therapy.
Luciano Costa, MD, PhD, spoke about how the results in the final primary analysis of the MASTER trial with daratumumab, carfilzomib, lenalidomide, and dexamethasone in multiple myeloma were not what he expected.

As the year 2021 comes to a close, CancerNetwork® sat down with Yael Cohen, MD, to discuss practice-changing clinical research in multiple myeloma that read out in 2021 and potential FDA updates that may take place in 2022.

Nina Shah, MD, discusses the current treatment options for treating patients with transplant-ineligible multiple myeloma.

Nina Shah, MD, on the current treatment options for treating patients with transplant-eligible multiple myeloma.

Nina Shah, MD, discusses the biggest news from the FDA for multiple myeloma in the past year.

Patients with triple-class relapsed/refractory multiple myeloma treated with ciltacabtagene autoleucel saw a greater survival benefit over physician's choice of treatment.

Most subgroups of patients with relapsed or refractory, heavily pretreated multiple myeloma showed durable responses at the 2-year follow-up to the CARTITUDE-1 trial.

Patients with heavily pretreated relapsed/refractory multiple myeloma achieved promising benefit in terms of cytokine release syndrome mitigation via double cycle 1 step-up dosing for cevostamab.

Deep responses were seen with single infusion ciltacabtagene autoleucel for heavily pretreated patients with multiple myeloma who were refractory to lenalidomide.

When asked about abstracts he thinks have the greatest potential to impact the standard of care in myeloma, Rafael Fonseca, MD, looked to emerging cellular therapies, specifically ciltacabtagene autoleucel for the treatment of heavily pretreated disease.
Patients with relapsed/refractory multiple myeloma continue to experience robust and durable benefit from treatment with ciltacabtagene autoleucel.

Patients with relapsed/refractory t(11;14) multiple myeloma appeared to benefit from treatment with venetoclax at a dose of 400 mg or 800 mg plus daratumumab/dexamethasone.

Luciano Costa, MD, PhD, spoke about the primary end point analysis for the MASTER trial examining daratumumab, carfilzomib, lenalidomide, and dexamethasone in multiple myeloma.

Daratumumab plus lenalidomide, bortezomib, and dexamethasone followed by transplant continues to show superior efficacy vs triplet therapy alone at a 2-year follow-up to the GRIFFIN trial.

A post hoc analysis showed that a dose reduction of selinexor yielded higher safety and efficacy for patients with multiple myeloma.