“Neoadjuvant endocrine therapy trials are feasible with relatively low toxicity,” Kelly K. Hunt, MD, FACS, FSSO, said during the 38th Annual Miami Breast Cancer Conference®.

Your AI-Trained Oncology Knowledge Connection!



“Neoadjuvant endocrine therapy trials are feasible with relatively low toxicity,” Kelly K. Hunt, MD, FACS, FSSO, said during the 38th Annual Miami Breast Cancer Conference®.

Allowing for the use of antiemetic prophylaxis and anti-diarrheal medication in the phase 3 KX-ORAX-001 study reduced study drug-related gastrointestinal adverse events.
Data focusing on patients seeking breast surgical procedures determined that broad skill sets are valuable for general surgeons practicing in rural areas, as breast surgeries only represented a small proportion of total surgical procedures conducted.

The addition of neratinib to capecitabine improved progression-free survival and central nervous system outcomes in patients with HER2-positive metastatic breast cancer with central nervous system disease.

More patients with early-stage estrogen receptor–positive breast cancer with high recurrence scores received chemotherapy following radiation in a single-institution study, and this resulted in a low recurrence rate across all patients examined.
“Our results support further study of serial molecular imaging along with combined [histone deacetylase inhibitors; HDACi] and [aromatase inhibitor; AI] therapy (such as ECOG-ACRIN study E2112), to further delineate the role of HDACi and potential biomarkers in AI-refractory ER+ advanced breast cancer,” wrote the study authors.

The developers of an oral formulation of paclitaxel will have to revisit efficacy and safety data in order to provide sufficient evidence for FDA approval.

Data examining 4 low-value breast cancer procedures found an association between facility-level characteristics and the use of these procedures, suggesting a need for de-implementation targeting efforts in various facilities.

A retrospective, population-based cohort study in JAMA Oncology found Black women in the United States were more likely to have a high-risk recurrence score and die of axillary node-negative breast cancer than non-Hispanic White women who had similar scores.

In the OlympiA trial, patients with high-risk HER2-negative breast cancer were randomized 1:1 to receive either olaparib or placebo for 12 months.

CancerNetwork®’s podcast dissected a review article published in the journal ONCOLOGY® on the 21-gene recurrence score in patients with node-positive breast cancer.
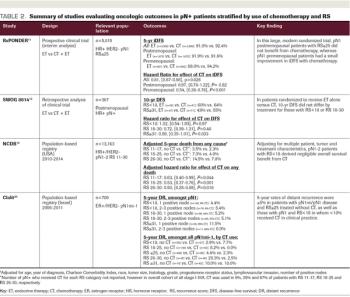
ABSTRACT The 21-gene Recurrence Score (RS) assay has been validated as both a prognostic and predictive tool in node-negative (pN0), estrogen receptor–positive (ER+), HER2-negative (HER2–) breast cancer. A large body of evidence supports the clinical utility of the RS in the node positive (pN+) population as well. Retrospective analyses of archived tissue from multiple clinical trials have found the RS to be prognostic in both endocrine therapy (ET)-treated and chemotherapy-treated patients with pN+ disease. Distribution of RS results in pN+ patients have also been consistent with those of pN0 populations. Data from the SWOG 8814 trial and large population-based registries further support the prognostic and potential predictive value of the RS. Specifically, patients with 1 to 3 positive nodes and RS less than 18 derived negligible benefit from adjuvant chemotherapy in these studies. In the prospective West German Study Group PlanB and ADAPT trials, pN+ patients with RS less than 11 and RS ≤25, respectively, who were treated with ET alone experienced excellent outcomes. Finally, 5-year results of the RxPONDER clinical trial randomizing patients with 1 to 3 positive nodes and RS ≤25 to ET alone vs ET plus chemotherapy confirmed an absence of chemotherapy benefit in postmenopausal patients. Clinical practice guidelines support use of the RS in the pN+, ER+/HER2– population, and many institutions have adopted the RS to guide clinical decision-making, resulting in a net reduction of adjuvant chemotherapy use. This review highlights the existing data supporting the prognostic and predictive ability of the RS in pN+ disease, current practice patterns related to RS use in this population, and emerging applications.






The combination of palbociclib and endocrine therapy demonstrated a more favorable safety profile and improved quality of life versus capecitabine among patients with metastatic breast cancer who were resistant to aromatase inhibitors, despite not improving progression-free survival.
Investigators suggested that the results of this study may assist health care providers in personalized therapeutic regimen selections for patients with early-stage breast cancer.

“We’re hearing about other antibody-drug conjugates, other agents in hormone receptor–positive metastatic disease, and the next generation of drugs that we’ll be using to treat our patients.”
Imagio, a diagnostic tool that uses novel technology to provide real-time information on suspicious lesions in the breast, is OK’d by the FDA.

The Breast Cancer Index assay is the only of its kind to be recommended in the National Comprehensive Cancer Network Guidelines for the treatment of breast cancer as being predictive of extended adjuvant endocrine therapy.

Investigators aimed to evaluate additional end points from the phase 3 RxPONDER trial in women with hormone receptor–positive, HER2-negative, lymph node–positive breast cancer.

In this trial, investigators launched RxPONDER, in which 5015 patients with a recurrence score between 0 and 25 were randomized to endocrine therapy alone or chemotherapy followed by endocrine therapy.