
Almost half of the resections performed for deep and malignant extremity soft-tissue sarcomas are done by non-oncology surgeons including orthopedic surgeons and general surgeons, according to a new study.

Your AI-Trained Oncology Knowledge Connection!


Almost half of the resections performed for deep and malignant extremity soft-tissue sarcomas are done by non-oncology surgeons including orthopedic surgeons and general surgeons, according to a new study.

Surgery is the mainstay of treatment for localized soft-tissue sarcoma (STS). It consists primarily of resection of the tumor along with a cuff of surrounding healthy tissue. In limb and trunk wall sarcomas, this basically implies resection of the surrounding soft tissues, which are mainly muscles, subcutaneous fat, and skin.[1] In the retroperitoneum, this necessarily should imply resection of adjacent viscera, even when they are not overtly involved.[2] This is the only way to avoid/minimize the presence of tumor cells at the cut surface (ie, positive microscopic surgical margins). Positive microscopic surgical margins are associated with a higher risk of local failure, distant metastases, and death.[3-6] Moreover, for STS located at critical sites, such as retroperitoneal sarcoma (RPS), positive surgical margins may have a direct impact on survival, favoring the development of inoperable local recurrences.[7] Indeed, unlike with STS arising in the extremities and trunk wall, local control in RPS poses a significant challenge and remains the leading cause of death, particularly in patients with low- to intermediate-grade tumors-roughly 75% of all cases.[8-13] Extending the resection to adjacent uninvolved viscera for primary RPS is the only way to minimize the presence of microscopic surgical margins and hence maximize the chance of cure. In essence, this strategy should often include ipsilateral nephrectomy and colectomy; locoregional peritonectomy and myomectomy (partial/total) of the muscle of the lateral/posterior abdominal wall (usually the psoas) (see Figure); splenectomy and left pancreatectomy, for tumors located on the left upper side; occasionally pancreaticoduodenectomy or hepatectomy, for tumors located on the right side; and vascular and bone resection only if vessels/bone are overtly infiltrated.[2]

Recently controversy has emerged regarding the extent of resection that constitutes optimal surgical management of retroperitoneal soft-tissue sarcoma.
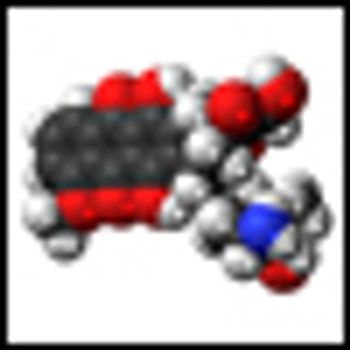
The addition of adjuvant chemotherapy using doxorubicin, ifosfamide, and cisplatin, to pelvic radiation therapy for the treatment of uterine sarcomas increased 3-year disease-free survival in a group of women with localized disease, but also resulted in two toxic deaths among the study group.
Decreasing the time between cycles of standard chemotherapy for Ewing’s sarcoma from 3 weeks to 2 weeks increased event-free survival without an associated increase in toxicity, according to the results of a Children’s Oncology Group report.
Despite its place in standard practice, the performance of pulmonary metastasectomy in patients with sarcoma and lung metastases may not improve the survival rate of these patients, according to information from a systematic review of studies investigating the procedure in this patient population.
Treatment of gastrointestinal stromal tumor (GIST) with regorafenib after prior treatment failure with both imatinib and sunitinib resulted in a PFS survival benefit for patients across all prespecified subgroups.
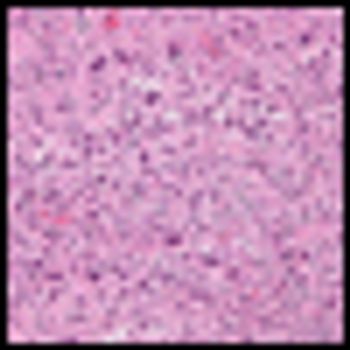
Results from a phase Ib/II trial presented at the ESMO 2012 Congress found that a tumor-targeting doxorubicin conjugate, aldoxorubicin (INNO-206), showed activity in relapsed soft-tissue sarcoma patients.
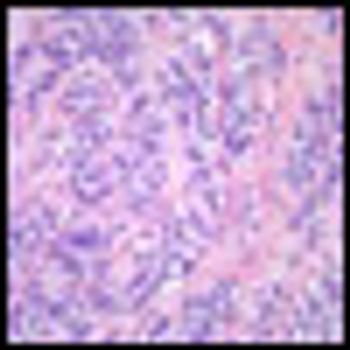
Treating resected grade II-III soft-tissue sarcoma with an adjuvant chemotherapy regimen of doxorubicin, ifosfamide, and lenograstim did not result in any increase in relapse-free survival or overall survival.

Earlier today the FDA approved pazopanib (Votrient) to treat patients with advanced soft-tissue sarcoma who have previously received chemotherapy. More than 20 subtypes of sarcoma were included in the clinical trial that led to the approval.
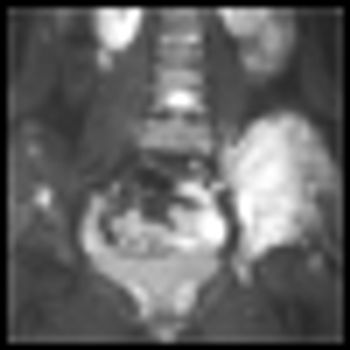
A combination of cixutumumab, a type 1 insulin-like growth factor receptor inhibitor, and temsirolimus, a mammalian target of rapamycin (mTOR) inhibitor, showed evidence of activity in refractory Ewing’s sarcoma tumors as well as small-round-cell tumors in a phase I multicenter clinical study.

Isolated pulmonary metastases (PM) represent a unique manifestation of the myriad presentations of systemic spread from a primary neoplasm.

In this issue of ONCOLOGY, Kon and Martin review the difficult issue of lung metastasis from sarcoma and its management.

Surgical resection of isolated pulmonary metastases has been incorporated into the management of cancer for more than 70 years. However, many questions still remain concerning indications, technique, and efficacy for this approach.

In this article, we provide an extensive review of patient selection criteria and surgical approaches, as well as of controversies regarding resection for metastatic sarcoma.

This rare cancer finally sheds its FDA designation as an orphan disease.

Using FDG-PET/CT, a multidisciplinary team has been able to determine within a short period of time whether neoadjuvant therapy for soft-tissue sarcoma is working.
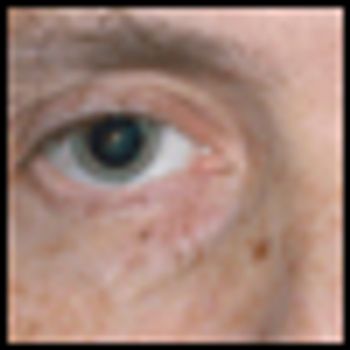
This case shows the importance of searching for antineural antibodies in oncologic patients with new neurologic deficits, and of having a judicious workup for occult malignancies in patients with known antineural antibodies.
Our case illustrates the fact that MDS-associated GS can be treated palliatively with radiation and hypomethylating agents in an appropriate setting. With the growing geriatric patient population, effective treatment options are needed in this disease.

Granulocytic sarcomas have been reported in nearly every part of the body, including the gastrointestinal (GI) and genitourinary (GU) tracts, central nervous system (CNS), and respiratory, lymphatic, and skeletal systems. A few case series have been reported through the years.

Several phase III clinical studies have shown significantly better results in treating certain cancers when hyperthermia therapy was added to radiation therapy, as compared to radiation treatments alone.

BSD Medical Corp announced that the results of a 340-patient randomized phase III clinical trial testing the benefit of adding hyperthermia therapy to chemotherapy were presented at the recent annual American Society of Clinical Oncology (ASCO) conference in Chicago.

The Oncologic Drugs Advisory Committee (ODAC) voted 12-to-2 not to recommend that FDA approve Junovan (mifamurtide, IDM Pharma) for treating newly diagnosed, resectable high-grade osteosarcomas in combination with chemotherapy following surgical resection.

Over the past 30 years, there has been a migration away from amputation and radical ablative surgical procedures and toward more conservative, function-preserving surgery combined with radiation to treat extremity and body wall soft-tissue sarcomas. Efforts are now being focused on optimizing and streamlining treatment, including identifying subpopulations of patients who may be adequately treated by surgery alone. The goal of these efforts is to minimize the risks for short- and long-term treatment-related morbidity while maintaining excellent rates of local tumor control. This report will briefly review the progress made in these areas.

Researchers have developed a two-gene test that can accurately distinguish between two common forms of gastrointestinal cancers.