Patients with VHL-mutant and wild type clear cell renal cell carcinoma may have comparable clinical characteristics, although other genetic alterations may be at play.

Your AI-Trained Oncology Knowledge Connection!


Patients with VHL-mutant and wild type clear cell renal cell carcinoma may have comparable clinical characteristics, although other genetic alterations may be at play.

A lack of understanding, communication, and more was expressed among both patients and caregivers regarding a subtype of kidney cancer.
Results of a phase 1/2 trial revealed the recommended phase 2 and maximum-tolerated dose of mobocertinib in Japanese patients with non–small cell lung cancer.
For patients with advanced EGFR Exon 20 Insertion–positive non–small cell lung cancer, mobocertinib appears to yield clinically meaningful activity compared with real-world data.
Findings from the phase 1b/2 MORPHEUS study indicated that patients with gastric and gastroesophageal junction cancer experienced limited responses to second-line linagliptin plus atezlolizumab.

Investigators identified a link between high tumor mutational burden and high responses to dostarlimab among patients with endometrial cancer.
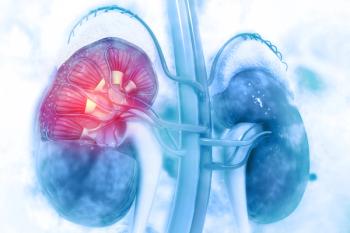
Patients with advanced renal cell carcinoma experienced improvements in both progression-free and overall survival after being treated with first-line immunotherapy vs sunitinib, although the benefit is yet to be determined in those with favorable-risk disease.

Further results from the KEYNOTE-426 trial continue to support the combination of pembrolizumab plus axitinib in patients with clear cell renal cell carcinoma.
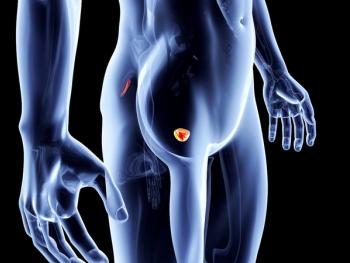
Results from the phase 2 ODEZA trial comparing patient preference of antiandrogen agents indicates benefits of darolutamide over enzalutamide in terms of certain cognitive functions in men with metastatic castration-resistant prostate cancer.
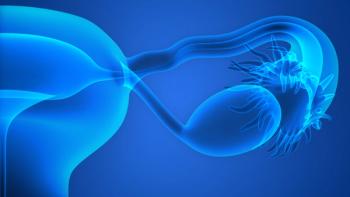
A subanalysis of the PRIMA/ENGOT-OV26/GOG-3012 trial found that quality of life according to patient-reported outcomes was not reduced despite treatment toxicities in those with ovarian, primary peritoneal, or fallopian tube cancer treated with niraparib versus placebo.
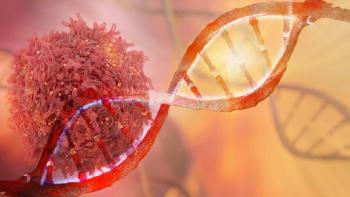
Results from the phase 1/2 ALEXANDER study found the investigational CAR T-cell product AUTO3 in combination with pembrolizumab to have a tolerable safety profile and elicit durable complete responses in patients with relapsed/refractory diffuse large B-cell lymphoma.
Post-operative radiotherapy was associated with a nonstatistically significant increase in disease-free survival in patients with completely resected stage IIIAN2 non-small cell lung cancer and therefore cannot be recommended as a standard of care.

The phase 3 IMagyn050/GOG 3015/ENGOT-OV39 trial indicated that the addition of atezolizumab to bevacizumab and chemotherapy failed to significantly improve progression-free survival in patients with newly diagnosed stage III/IV ovarian cancer.

Sotorasib showed promising antitumor activity in patients with non-small cell lung cancer that harbor the KRAS p.G12C mutation.
Balstilimab as a single agent and combined with zalifrelimab demonstrated promising objective response rates, regardless of PD-L1 expression, and a tolerable safety profile in patients with recurrent or metastatic cervical cancer.
“Results from this study suggest that tisotumab vedotin has the potential to be a new therapy for patients with previously treated recurrent and/or metastatic cervical cancer,” said lead study author Robert L. Coleman, MD, FACOG, FACS.
Hepatic arterial infusion chemotherapy with oxaliplatin, fluorouracil, and leucovorin significantly improved overall survival compared with transarterial chemoembolization in patients with unresectable hepatocellular carcinoma.
The addition of nivolumab (Opdivo) to chemotherapy resulted in a statistically significant improvement in PFS and evoked higher ORRs in patients with previously untreated advanced or recurrent gastric and gastroesophageal junction cancer.

Findings from the noncomparative, phase 2, biomarker-driven BIONIKK trial support the use of molecularly-directed frontline therapy as means to enrich responses in patients with metastatic clear cell renal cell carcinoma.
The combination of nivolumab (Opdivo) and chemotherapy led to a statistically significant survival benefit among previously untreated patients with PD-L1–positive advanced gastric cancer, gastroesophageal junction cancer, and esophageal adenocarcinoma.

The study found that that weekly dose-dense chemotherapy is not superior to standard 3 weekly chemotherapy for patients with epithelial ovarian cancer with regard to progression-free survival and overall survival.
Findings from the phase 2 FLIPPER trial indicated that frontline fulvestrant (Faslodex) in combination with palbociclib (Ibrance) demonstrated an improvement in PFS at 1 year in patients with endocrine-sensitive HR-positive, HER2-negative metastatic breast cancer.
“The combination of cabozantinib and atezolizumab demonstrated encouraging clinical activity in previously untreated patients with advanced ccRCC,” said investigator Sumanta Kumar Pal, MD.

Based on these data, researchers indicated that adjuvant osimertinib would be an effective and practice-changing treatment in this setting.
Frontline pembrolizumab (Keytruda) plus chemotherapy significantly improved overall survival, progression-free survival, and objective response rates compared with chemotherapy alone in patients with locally advanced unresectable or metastatic esophageal cancer.

Patients with locally advanced or metastatic urothelial carcinoma receiving enfortumab vedotin had increased overall survival at 12- and 18-month intervals.

The addition of tucatinib to trastuzumab and capecitabine in patients with HER2-positive metastatic breast cancer with and without brain metastases resulted in statistically significant and clinically meaningful improvements in progression-free and overall survival.
Treatment with a novel BiTE® immuno-oncology therapy showed a manageable safety profile with preliminary efficacy in patients with metastatic castration-resistant prostate cancer.

In patients with metastatic castration-resistant prostate cancer (mCRPC) with PTEN loss, ipatasertib combined with abiraterone acetate (Zytiga) plus prednisone led to a significantly superior radiographic progression-free survival and antitumor activity.
Apatinib in combination with gefitinib in the first-line setting demonstrated superior progression-free survival (PFS) in patients with advanced EGFR-mutant non–small cell lung cancer.