Lymphoma
Latest News

CAR T-Cell Products Yield Notable Responses Without Increase in Neurotoxicity or CRS in LBCL With CNS Involvement
Latest Videos
CME Content
More News
Patients with high-risk large B-cell lymphoma experienced potent, long-term responses following treatment with axicabtagene ciloleucel in the first line.
Frederick Lock, MD, spoke about how future research regarding axicabtagene ciloleucel for patients with large B-cell lymphoma will progress.
The phase 1/2 TakeAim Lymphoma trial examining emavusertib for patients with B-cell malignancies has received a partial clinical hold pending review by the FDA.

This review provides an updated analysis of the literature and discusses the authors' approach to the diagnosis and treatment of patients with marginal zone lymphoma.

Investigators reported topline findings from the phase 1/2 EPCORE NHL-1 trial assessing epcoritamab in patients with relapsed/refractory large B-cell lymphoma.

Frederick Lock, MD, spoke about how axicabtagene ciloleucel has improved survival in patients with large B-cell lymphoma.

Ibrutinib plus venetoclax given at a fixed duration yielded favorable progression-free survival in patients with previously untreated, high-risk chronic lymphocytic leukemia and small lymphocytic lymphoma.

The European Commission approved lisocabtagene maraleucel for the treatment of certain patients with relapsed/refractory large B-cell lymphoma.

Biomarker analysis and studies of pharmacokinetics, pharmacodynamics, and T-cell composition show certain T-cell characteristics are linked with outcomes and toxicity with axicabtagene ciloleucel in large B-cell lymphoma.

Investigators of a phase 1/2 trial observed positive response outcomes with expanded natural killer cells plus AMF13 in patients with heavily pretreated lymphoma.

Frederick Lock, MD, spoke about the approval of axicabtagene ciloleucel and how it’s beneficial for patients with large B-cell lymphoma who were treated with frontline chemoimmunotherapy.

Updated data from a pivotal trial that led to the approval of zanubrutinib for the treatment of patients with relapsed or refractory mantle cell lymphoma appears to highlight persistent benefit.

Based on results of the ZUMA-7 trial, the FDA approved axicabtagene ciloleucel for the treatment of certain patients with large B-cell lymphoma who received chemoimmunotherapy in the frontline setting.

The FDA supported submission of application for approval of zandelisib in follicular lymphoma and other indolent non-Hodgkin lymphomas based on the phase 3 COASTAL trial.

Patients with mantle cell lymphoma appeared to benefit from brexucabtagene autoleucel as salvage therapy in the real-world setting.

Phase 2 data highlighted a high complete response rate and undetectable minimal residual disease rate in patients with indolent clinical forms of mantle cell lymphoma who received ibrutinib plus rituximab.

Younger survivors of B-cell non-Hodgkin lymphoma appeared to have an increased risk of adverse health outcomes 5 or more years after diagnosis compared with older survivors.

The recommended dose of ceritinib once daily with food demonstrated promising preliminary clinical activity in patients with ALK-positive relapsed/recurrent inflammatory myofibroblastic tumors and anaplastic large cell lymphoma, and certain subsets of relapsed/refractory neuroblastoma.
The CD19-directed CAR T-cell therapy lisocabtagene maraleucel was granted priority review by the FDA following an application for its use in patients with relapsed or refractory large B-cell lymphoma receiving therapy in the second-line setting.

Jonathan R. Day, MD, PharmD, and Brian K. Link, MD, give their perspective on emerging treatments for follicular lymphoma.
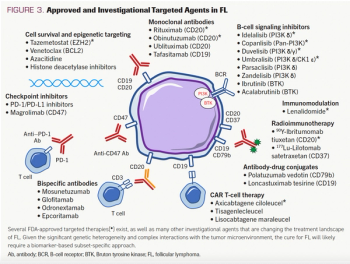
This review article written by Kirk E. Cahill, MD, and Sonali M. Smith, MD, discusses standard and emerging therapies for the treatment of follicular lymphoma.

Amitkumar Mehta, MD, detailed encouraging data presented at the 63rd ASH Annual Meeting for treating mantle cell lymphoma.

Michael R. Bishop, MD, discussed how he thinks the results from phase 3 BELINDA study may impact the treatment landscape in B-cell lymphomas moving forward.

Research from a 2-part, phase 2 trial found that ibrutinib and rituximab followed by hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone produced positive response data and a manageable safety profile for younger patients with mantle cell lymphoma.
Umbralisib is currently under investigation by the FDA regarding a potentially increased risk of death in patients with lymphomas following data from an ongoing trial investigating its use in chronic lymphocytic leukemia.