
Everolimus plus lanreotide elicited a PFS of 29.7 months compared with 11.5 months from everolimus monotherapy in patients with gastroenteropancreatic neuroendocrine tumors.

Your AI-Trained Oncology Knowledge Connection!


Everolimus plus lanreotide elicited a PFS of 29.7 months compared with 11.5 months from everolimus monotherapy in patients with gastroenteropancreatic neuroendocrine tumors.

Sessions of interest at the 2025 Gastrointestinal Cancers Symposium will include data on colorectal cancer, pancreatic ductal adenocarcinoma, and more.

Jun Gong, MD, and Daneng Li, MD, spoke about trials they feel can impact the gastrointestinal space during a CancerNetwork® X Space.
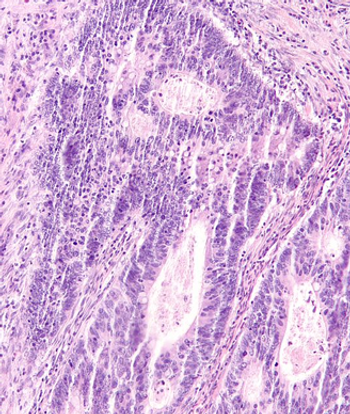
Results from the randomized LASRE trial support the use of laparoscopic-assisted surgery for patients with low rectal cancer.
Investigators note that early interventions such as nutritional support and prehabilitation require further investigation to increase both the quality of life and overall survival for patients with esophageal cancer.
Adjuvant chemotherapy does not yield an improvement in ctDNA clearance compared with observation among patients with stage II colon cancer in the phase 2/3 COBRA trial.
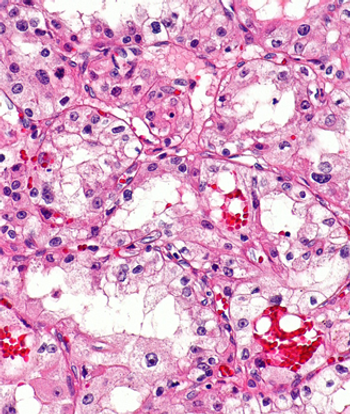
Disease-free survival benefits with chemotherapy appears to be more prominent in patients with colorectal cancer who are minimal residual disease–positive compared with those minimal residual disease–negative disease.
Neoadjuvant botensilimab plus balstilimab appears to be safe and effective in patients with colorectal cancer regardless of mismatch repair status in the phase 2 NEST-1 trial.

Real-world data suggest a role for noncytotoxic chemotherapy treatments such as EGFR inhibitors beyond the frontline setting for patients with metastatic colorectal cancer.
Duration of response results with pembrolizumab plus lenvatinib in advanced hepatocellular carcinoma appear to be ‘promising’ in the phase 3 LEAP-002 trial.

Results from the phase 3 CheckMate-8HW trial highlight that the safety of frontline nivolumab plus ipilimumab in microsatellite instability–high or mismatch repair deficient is comparable with prior reports.

Real-world data may elucidate the characteristics and factors that influence long-term remission with regorafenib among patients with metastatic colorectal cancer.

Data from the phase 3 NETTER-2 trial support the frontline use of Lutetium Lu 77 dotatate well-differentiated gastroenteropancreatic neuroendocrine tumors.

Data from the phase 2 NAVIGATE trial support the wider adoption of next-generation sequencing panels including NTRK gene fusions in the treatment of those with gastrointestinal cancers.
Durvalumab plus bevacizumab and TACE may be a new standard of care in unresectable hepatocellular carcinoma eligible for embolization, according to Riccardo Lencioni, MD.

Patients with hepatocellular carcinoma and Child-Pugh-B liver function appear to have worse overall survival following treatment with regorafenib in the REFINE trial.

Treatment-emergent adverse effects following therapy with fostrox plus lenvatinib among those with hepatocellular carcinoma appear to be manageable in a phase 1a/2a study.

Investigators observe a benefit with durvalumab plus neoadjuvant chemotherapy among subgroups of patients regardless of microsatellite instability status in the phase 3 MATTERHORN trial.

Findings from the phase 3 FRESCO-2 trial support fruquintinib’s potential to provide an improved survival benefit and quality of life for those with previously treated metastatic colorectal cancer.
The DeFianCe trial is using DKN-01 plus bevacizumab and chemotherapy to determine if a clinical benefit would occur in patients with microsatellite stable colorectal adenocarcinoma.
Data from the phase 3 SKYSCRAPER-08 trial may support tiragolumab plus atezolizumab and chemotherapy as an alternative frontline treatment option for those with locally advanced or metastatic esophageal squamous cell carcinoma.

Combining ASKB589 with capecitabine, capecitabine, and sintilimab leads to no treatment discontinuation due to adverse effects among patients with gastric or gastroesophageal junction cancer in a phase 1/2 trial.

In the overall population and in patients with a PD-L1 CPS of 5 or greater who received nivolumab plus chemotherapy, overall survival and progression-free survival improved when compared with chemotherapy alone.

Results from an observational study found nivolumab plus chemotherapy helped enhance overall survival and progression-free survival for patients with advanced or metastatic gastric cancer, gastroesophageal junction cancer, or esophageal adenocarcinoma.

An expert from the University of California, Los Angeles suggests that the NALIRIFOX may be a beneficial treatment for patients with pancreatic ductal adenocarcinoma.

Patients with metastatic pancreatic ductal adenocarcinoma who receive NALIRIFOX tend to have more gastrointestinal toxicity while nab-paclitaxel/gemcitabine results in more cytopenias, according to an expert from University of California, Los Angeles.

An expert from the University of California, Los Angeles described the purpose and design of the phase 3 NAPOLI-3 study, assessing NALIRIFOX in metastatic pancreatic ductal adenocarcinoma.

Findings from a phase 2 trial indicate that CTX-009 plus paclitaxel may produce an encouraging responses in patients with advanced biliary tract cancer, according to investigators.

Findings from the phase 3 BREAKWATER study indicate that encorafenib plus cetuximab and chemotherapy produces activity and is well-tolerated in metastatic colorectal cancer harboring BRAF V600E mutations.

Findings from the phase 2 MOUNTAINEER trial indicate that HER2 scoring algorithms may help in identifying which patients are suitable to receive tucatinib plus trastuzumab for metastatic colorectal cancer.