
Investigators behind a phase 1 trial investigating MT-0169 in relapsed or refractory multiple myeloma and non-Hodgkin lymphoma will pause enrollment of new patients following prior reports of cardiac adverse effects in 2 patients.

Your AI-Trained Oncology Knowledge Connection!


Investigators behind a phase 1 trial investigating MT-0169 in relapsed or refractory multiple myeloma and non-Hodgkin lymphoma will pause enrollment of new patients following prior reports of cardiac adverse effects in 2 patients.

Tavokinogene telseplasmid plus pembrolizumab is reported to be well tolerated in patients with advanced, pre-treated melanoma based on data from the phase 2 KEYNOTE-695 trial.
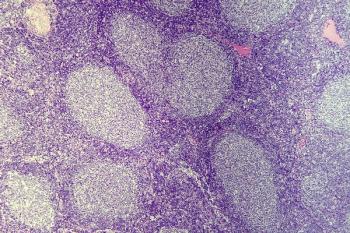
Primary outcome data from phase 3 confirmatory studies evaluating ibrutinib in mantle cell lymphoma and marginal zone lymphoma are insufficient to support conversion to full FDA approval in these indications.

SynKIR-110, which received fast track designation from the FDA, is currently under investigation as treatment for patients with mesothelioma in the phase 1 STAR-101 trial.
Patients with advanced high-grade epithelial BRCA mutation–negative ovarian cancer appear to benefit from treatment with olaparib and durvalumab plus bevacizumab and chemotherapy.

Farshid Sadeghi, MD, elaborates on improvements in minimally invasive surgery technique and the interdisciplinary cooperation that goes into treating kidney cancer.

Collaboration in kidney cancer palliative care and integrative medicine programs underscores the current development of University Hospitals’ integrative oncology wing.

Data from a phase 1/2 dose escalation study investigating BDC-10001 plus nivolumab in HER2-expressing tumors support the advancement of additional phase 2 trials evaluating the novel antibody conjugate.
Findings from 2 phase 3 trials support the recommendation to approve sodium thiosulfate to reduce the risk of cisplatin-related hearing loss in pediatric patients with solid tumors in Europe.

Factors including ARID1A mutations and tumor mutational burden appear to correlate with progression-free survival and overall survival following immunotherapy for advanced bladder cancer.

Experts in the multiple myeloma space review available therapies such as CAR T-cell therapy, bispecifics, and B-cell maturation antigen–targeted agents for patients with multiple myeloma.

The addition of radiation to chemotherapy did not improve overall survival in patients with advanced endometrial cancer, according to an expert from Northwestern Medicine.

Findings from the phase 3 TRANSFORM study support the Committee for Medicinal Products for Human Use’s recommendation to approve lisocabtagene maraleucel for large B-cell lymphoma.
The safety profile seen in the phase 3 CONTACT-03 trial, assessing cabozantinib and atezolizumab in advanced renal cell carcinoma, is consistent with previous findings.
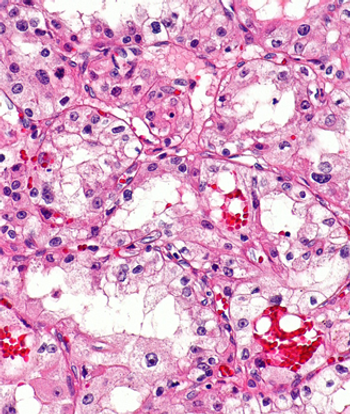
Progression-free survival and overall survival appear to be worse in patients with metastatic colorectal cancer who have a high- vs low-BRAF allele fraction after treatment with a BRAF inhibitor plus an anti-EGFR agent plus or minus a MEK inhibitor.

The FDA’s decision to lift a partial clinical hold on the phase 1/2 VELA trial follows reports of visual adverse effects in patients with advanced solid tumors in February 2023.

Experts discuss findings presented at ASH 2022 regarding chronic lymphocytic leukemia, and how they can be applied to clinical practice.

Ravi Vij, MD, MBA, and Luciano Costa, MD, face off over myeloma data from ASH 2022.
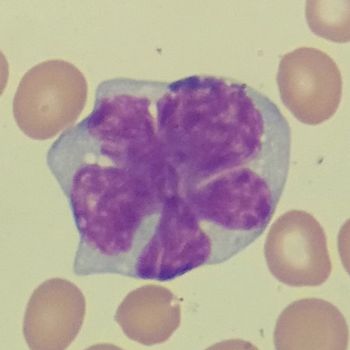
Retrospective data encourage further exploration of non-ASCT regimens in younger patients with mantle cell lymphoma, while maintenance rituximab should be considered following first-line therapy with bendamustine plus rituximab.

The FDA has set a Prescription Drug User Fee Act date in the fourth quarter of 2023 for encorafenib plus binimetinib as a treatment for BRAF V600E–mutant metastatic non–small cell lung cancer.

A panel of experts from Moffitt and Mayo discuss newly published data from the 2022 American Society of Hematology (ASH) Annual Meeting and Exposition.

Nazy Zomorodian, NP, spoke with CancerNetwork® about the unique dosing schedule of enfortumab vedotin plus pembrolizumab following its accelerated approval by the FDA in advanced/metastatic urothelial cancer.
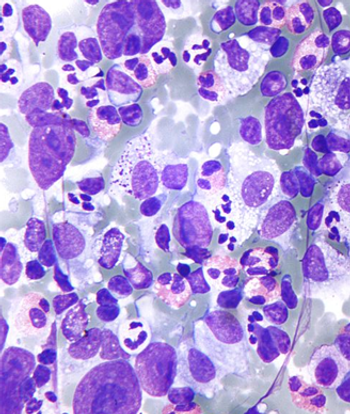
Acceptance of an investigational new drug application for GRC 54276 will allow investigators to proceed with a phase 1/2 trial evaluating the agent in patients with advanced solid tumors and lymphomas.
Data from a Chinese phase 1/2 trial suggest that osemitamab monotherapy may yield prolonged responses in Claudin18.2-expressing pancreatic cancer.

A first-in-human phase 1/2a trial will assess anti-PAUF monoclonal antibody PBP1510 in advanced/metastatic pancreatic cancer.

Results from cohort K of the phase 1b/2 KEYNOTE-869 trial led to the accelerated approval of enfortumab vedotin plus pembrolizumab in patients with locally advanced or metastatic urothelial cancer who cannot receive cisplatin-based chemotherapy.

In addition to granting fast track designation to RRx-001, the FDA accepts an investigational new drug application for the agent to reduce and/or prevent radiation- and chemotherapy-associated oral mucositis.

The new drug application that was submitted to the FDA for fruquintinib in refractory, metastatic colorectal cancer is supported by data from the phase 3 FRESCO-2 trial.

Patients with non–small cell lung cancer who had mild/moderate immune-related adverse effects appear to have improved overall survival compared with those without following atezolizumab-based therapy.

An expert from Dana-Farber Cancer Institute indicates that urologists should refer patients with prostate cancer who present with multiple high-risk factors at surgery to a radiation and medical oncologist.