
Investigators at Memorial-Sloan Kettering plan to tease out exactly how much vitamin D is enough to improve survival prospects.

Your AI-Trained Oncology Knowledge Connection!


Investigators at Memorial-Sloan Kettering plan to tease out exactly how much vitamin D is enough to improve survival prospects.

Patients saw improvements in progression-free survival, while oncologists saw benefit of evidence-driven medicine at work.

Current guidelines may be inadequate in at-risk African-American and Latino populations.

Early detection of cancer and novel chemotherapy agents have resulted in longer survival following a colorectal cancer diagnosis.

Metabolic inhibition by chemotherapeutic drugs most likely hampers imaging results.

Every year in the United States, approximately 160,000 cases of colorectal cancer (CRC) are diagnosed, and about 57,000 patients die of the disease, making it the second leading cause of death from cancer among adults.

In this ASCO podcast, Dr. Goldberg, Distinguished Professor, Hematology/Oncology, at the University of North Carolina Lineberger Comprehensive Cancer Center and the Physician-in-Chief of the North Carolina Cancer Hospital, spoke about the new developments in advanced colorectal cancer since his ASCO 2003 practice-changing presentation.

A high intake of dietary polyamines, which are commonly found in orange juice, corn, red meat, peas, and nuts, has been linked to the size and grade of colorectal adenomas found on screening colonoscopy.

Confusion over important prognostic indicator may lead to unnecessary treatment.

The combination of oxaliplatin plus fluorouracil/leucovorin is known as the FOLFOX regimen, and it has become a standard regimen for colorectal cancer (CRC), both as adjuvant therapy and as treatment for metastatic disease. Unfortunately, platinum-based chemotherapies also produce neurotoxicity as a side effect. Neurotoxicity is the most common dose-limiting toxicity of oxaliplatin, and it is one of the major causes for patients to stop receiving chemotherapy. It can manifest as either of two distinct syndromes: a transient, acute syndrome that can appear during or shortly after the infusion (~1%–2% of patients), and a dose-limiting, cumulative sensory neuropathy. Calcium/magnesium (Ca/Mg) infusions have been used to decrease the incidence of oxaliplatin-induced neuropathy. The actual utility of Ca/Mg infusions in this setting has been an interesting and controversial topic. They may reduce the severity of neurotoxicity, but some investigators have questioned whether they also will alter the efficacy of these chemotherapy regimens. In this paper, we review the clinical data concerning the usefulness of Ca/Mg infusions in reducing the incidence of oxaliplatin-induced neuropathy as well as their effect on responsiveness to chemotherapy.

Four years ago, at least five years of statin use was found to be associated with a 53% reduction in the risk of colorectal cancer and that association still stands, according to a coauthor of one of the initial studies to make the statin-cancer connection.

A primer on managing the chemorefractory patient from Eric Van Cutsem, MD, PhD.

In this supplement to Oncology, guest editor Axel Grothey from the Mayo Clinic, explores adjuvant therapy considerations in stage II colon cancer from different angles.

In the treatment of colon cancer today, the decision-making involved in the treatment of stage II disease is probably the most challenging aspect. The major question is whether or not these patients should receive postoperative adjuvant chemotherapy. Approximately 75% of stage II colon cancer is cured by surgery alone. For the remaining 25% of cases, there is great debate over whether adjuvant chemotherapy is sufficiently effective in enough patients to warrant the exposure to potentially toxic treatments. In the important QUASAR clinical trial, stage II patients were randomized to either fluorouracil (5-FU)-based therapy or observation. The results demonstrated an approximate 3% improvement in outcome for the 5-FU–treated patients. This leads to the assumption that treating all stage II patients with adjuvant chemotherapy is gross overtreatment, when essentially 97% of these patients will not benefit. Clearly the only way to approach this decision is through risk determination. In this article, I will describe the current state of defining high- and low-risk disease, which is mainly through histopathologic characteristics, as well as discuss emerging approaches such as molecular markers and genomic profiling

it is obvious that some patients with stage II cancers have excellent outcomes and do not require additional therapy, whereas other patients have cancers that biologically behave more like stage III tumors.Over the past years, our understanding of stage II colon cancer has made significant advances leading to a refinement in the identification of the patient population and the treatment options considered appropriate as adjuvant therapy in this setting.

Colon cancer is estimated to have accounted for 106,100 new cancer cases and 49,920 cancer-related deaths in 2009. Over half of these new diagnoses and deaths occur in individuals age 70 and older.

Approximately 150,000 new cases of colorectal cancer were expected for the year 2009 in the United States. Moreover, 49,920 deaths related to colorectal cancer were also predicted for the same year. The age-adjusted cancer death rates related to colorectal cancer have steadily declined over the past 2 decades. This improvement is a direct consequence of advances in prevention and treatment, including colorectal cancer screening, diagnostic tests, surgical technique, adjuvant therapies, and medical support.

In this issue of ONCOLOGY, Dr. Czito and colleagues from Duke University School of Medicine and the University of Texas Southwestern describe the potential benefit of incorporating intensity-modulated radiation therapy (IMRT) into the combined-modality treatment of anal canal cancer.[1] As the authors well delineate, the treatment of anal canal cancer has progressed from radical surgery to organ preservation with the use of definitive chemoradiotherapy.
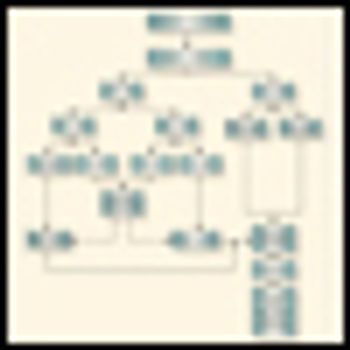
The combined-modality care of the patient with colon or rectal cancer metastatic to the liver demands a team approach. It is little wonder that there is much confusion about this topic, given the number of unique treatment options that are delivered in a sequential and reiterative process. The concept of multidisciplinary approaches to complex cancer challenges has been adopted for a variety of tumor types and situations.
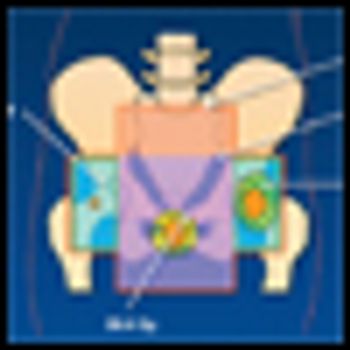
Historically, the treatment of squamous cell carcinoma of the anal canal has been an abdominoperineal resection (APR), resulting in loss of the anus and rectum with need for a permanent colostomy.

BERLIN-The failure of a major colon cancer trial to reach its primary endpoint surprised even the most seasoned gastrointestinal cancer investigators. Overall survival was not improved when cetuximab (Erbitux) was added to a first-line oxaliplatin-based regimen (Eloxatin), according to phase III COIN trial at ECCO/ESMO 2009.

The treatment of older patients with colorectal cancer is not always straightforward. As highlighted in the article by Dr. Ades, the heterogeneity of physiologic aging, the increasing prevalence of comorbid disease with age, and changing preferences with aging make counseling about adjuvant therapy more complex for older patients than for younger patients.

States population will be over 65 years old, with 2% of the population over 84. The corresponding projections for 2050 are 21% and 5%, respectively.[1] These projections underscore the aging of the population, with most recent estimates of life expectancy hitting a record high of 78.1 years.[2] With Americans living longer than ever before, physicians are already seeing larger numbers of elderly patients with cancers whose incidence increases with age, including colon cancer.

The Hippocratic principle of not harming the patient has remained up to this day an undisputed dogma in medicine. It reminds the physician of the possible detrimental, if not lethal, outcome of the treatment he prescribes and implicitly enforces good medical practice, although the true impact will unlikely be known. Oncology is one subspecialty of Medicine where this dilemma-ie, the pros and cons of treatment-is continuously put to the test, as the physician must decide on treatment for an often life-threatening illness while taking into account individual factors such as the patient’s will, performance status, available standard treatment options, and possible experimental approaches.

Davies/Goldberg Article Reviewed. The past decade has seen exciting developments in the field of colorectal cancer, particularly in the setting of advanced disease.