
Roche/Genentech releases early results from AVANT trial.

Your AI-Trained Oncology Knowledge Connection!


Roche/Genentech releases early results from AVANT trial.

In a large study of resected pancreatic cancer, overall survival did not differ whether patients received gemcitabine or 5-FU/folinic acid.

Despite efforts by the manufacturer to reduce the cost of Avastin, the clinical effectiveness agency for England and Wales says that it will not recommend the drug for the first-line treatment of metastatic colorectal cancer.

Primary surgery with an abdominoperineal resection (APR) was historically the standard of care for localized anal squamous cell carcinoma. APR achieved 40%-70% survival rates at five years, with local failures from 27%-47%.[1,2] With modern technology and radiation dose escalation, external beam radiation therapy (EBRT) studies have improved complete response rates, decreased morbidity, and improved sphincter preservation rates. Nigro et al added 5-fluorouracil (5FU) and mitomycin C (MMC) to concurrent EBRT [3,4] and impressive complete response rates inspired other groups to investigate the role of chemotherapy as a component of sphincter-preserving therapy. The European Organization for Research and Treatment of Cancer (EORTC) and United Kingdom Coordinating Committee on Cancer Research (UKCCCR) studies reported improved local control and colostomy-free survival when chemotherapy (5FU/MMC) was administered in conjunction with radiation.[5,6] The five-year survival rate for patients receiving standard chemoradiation approaches 70%; however, 20%-40% experience grade 3-4 toxicity, and administration with MMC causes profound hematologic toxicity.

The treatment of cancer of the anal canal has changed significantly over the past several decades. Although the abdominoperineal resection (APR) was the historical standard of care, a therapeutic paradigm shift occurred with the seminal work of Nigro, who reported that anal canal cancer could be treated with definitive chemoradiation, with APR reserved for salvage therapy only. This remains an attractive approach for patients and physicians alike and the standard of care in this disease. Now, nearly four decades later, a similar approach continues to be utilized, albeit with higher radiation doses; however, this strategy remains fraught with considerable treatment-related morbidities. With the advent of intensity-modulated radiation therapy (IMRT), many oncologists are beginning to utilize this technology in the treatment of anal cancer in order to decrease these toxicities while maintaining similar treatment efficacy. This article reviews the relevant literature leading up to the modern treatment of anal canal cancer, and discusses IMRT-related toxicity and disease-related outcomes in the context of outcomes of conventionally treated anal cancer.

Early detection of cancer and novel chemotherapy agents have resulted in longer survival following a colorectal cancer diagnosis.

Therapeutic outcomes for patients with most of the cancers originating in the upper gastrointestinal track still remain disappointing.

In this ASCO podcast, Dr. Goldberg, Distinguished Professor, Hematology/Oncology, at the University of North Carolina Lineberger Comprehensive Cancer Center and the Physician-in-Chief of the North Carolina Cancer Hospital, spoke about the new developments in advanced colorectal cancer since his ASCO 2003 practice-changing presentation.

Dr. Abbas and colleagues delineate the current status of chemoradiation for anal carcinoma. Their thorough and thoughtful review serves as an excellent summation of the current therapeutic approach of the past few years.

The treatment of anal squamous cell cancer with definitive chemoradiation is the gold-standard therapy for localized anal cancer, primarily because of its sphincter-saving and colostomy-sparing potential.

Dr. Fakih and colleagues provide a detailed and thoughtful review of the role of chemoradiation in anal cancer treatment. They have included a comprehensive description of the epidemiology and risk factors for the development of squamous cell carcinoma of the anal canal, including the strong association with human papillomavirus (HPV) infection and increased incidence in human immunodeficiency virus (HIV)-positive individuals.

Although anal cancer is a rare disease, its incidence is increasing in men and women worldwide. The most important risk factors are behaviors that predispose individuals to human papillomavirus (HPV) infection or immunosuppression. Anal cancer is generally preceded by high-grade anal intraepithelial neoplasia (HGAIN), which is most prevalent in human immunodeficiency virus (HIV)-positive men who have sex with men. There is a general consensus that high-risk individuals may benefit from screening. Meta-analysis suggests that 80% of anal cancers could be avoided by vaccination against HPV 16/18. Nearly half of all patients with anal cancer present with rectal bleeding. Pain or sensation of a rectal mass is experienced in 30% of patients, whereas 20% have no tumor-specific symptoms. According to the Surveillance Epidemiology and End Results (SEER) database, 50% of patients with anal cancer have disease localized to the anus, 29% have regional lymph node involvement or direct spread beyond the primary, and 12% have metastatic disease, while 9% have an unknown stage. Clinical staging of anal carcinoma requires a digital rectal exam and a computed tomography scan of the chest, abdomen, and pelvis. Suspicious inguinal lymph nodes should be subject to pathologic confirmation by fine-needle aspiration. The 5-year relative survival rates are 80.1% for localized anal cancer, 60.7% for regional disease, and 29.4% for metastatic disease. Part 2 of this two-part review will address the treatment of anal cancer, highlighting studies of chemoradiation.

Confusion over important prognostic indicator may lead to unnecessary treatment.

A novel IGF-receptor inhibitor stabilized disease in a majority of pancreatic cancer patients, according to a report at the 2010 GI Cancers Symposium.

Four years ago, at least five years of statin use was found to be associated with a 53% reduction in the risk of colorectal cancer and that association still stands, according to a coauthor of one of the initial studies to make the statin-cancer connection.

A primer on managing the chemorefractory patient from Eric Van Cutsem, MD, PhD.

Screening is the key to protecting those at risk for pancreatic cancer, according to presenters at the 2010 Gastrointestinal Cancers Symposium.

Fluorouracil, oxaliplatin (Eloxatin), and bevacizumab (Avastin) for first-line therapy is preferred in metastatic colon cancer.

This review summarizes the current data on efficacy and rationale of adjuvant treatment for hepatocellular carcinoma (HCC) after orthotopic liver transplantation (OLT). The authors review prognostic factors for disease recurrence and adjuvant therapy after OLT, including systemic chemotherapy, intra-arterial chemoembolization, immunosuppressant effects, and sorafenib (Nexavar). Several interesting questions are raised in the article, including: (1) When is the best time to apply systemic chemotherapy?

In this issue of ONCOLOGY, Kim et al discuss adjuvant therapy after liver transplantation to decrease recurrence of hepatocellular carcinoma (HCC). Liver transplantation offers the best overall and recurrence-free survival for the treatment of stage I and II HCC. The landmark study in 1996 by Mazzaferro demonstrated that liver transplantation of patients with one lesion less than 5 cm or with up to three lesions but all less than 3 cm (the Milan criteria) resulted in low recurrence rates and similar survival to patients without HCC.[1]

BERLIN-A pair of colon cancer trials using panitumumab (Vectibix) not only proved the efficacy of the drug in patients with nonmutated KRAS but also highlighted the importance of ascertaining KRAS status. Trial 181 evaluated panitumumab in combination with FOLFIRI as a second-line treatment for metastatic colorectal cancer, while trial 203 paired the anti-EGFR agent with FOLFOX4 as first-line treatment.

Annick Van den Abbeele, MD, couldn’t believe her eyes. Dr. Van den Abbeele, the chief of radiology at Boston’s Dana-Farber Cancer Institute, had seen the patient just a month earlier. At that time, the 35-year-old woman had a gastrointestinal stromal tumor in her abdomen that was so large, she looked six months’ pregnant. But at the patient’s follow up FDG-PET study, the tumor was completely gone.

Dr. Czito and colleagues provide an intriguing overview on adapting and using more technically advanced techniques to deliver radiation therapy for anal cancer patients. The paper starts with a brief history of the treatment of anal cancer, moving from abdominoperineal resection to combined-modality therapy with radiation and chemotherapy and discusses the trials showing that combined chemoradiotherapy is superior to radiation alone in terms of local control and colostomy-free survival.[1,2] Adding mitomycin to fluorouracil (5-FU) has been scrutinized for increasing toxicity but has been shown to decrease colostomy rates compared to cisplatin/5-FU or 5-FU alone.[3,4]
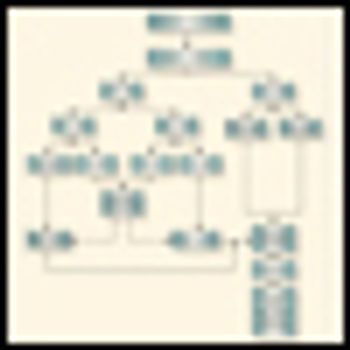
The combined-modality care of the patient with colon or rectal cancer metastatic to the liver demands a team approach. It is little wonder that there is much confusion about this topic, given the number of unique treatment options that are delivered in a sequential and reiterative process. The concept of multidisciplinary approaches to complex cancer challenges has been adopted for a variety of tumor types and situations.

The management of patients with colorectal cancer that has metastasized to the liver is a common clinical problem