A new human papillomavirus (HPV) vaccine that protects against nine types of HPV and would protect against about 90% of cervical cancers could be available in 2015.

Your AI-Trained Oncology Knowledge Connection!


A new human papillomavirus (HPV) vaccine that protects against nine types of HPV and would protect against about 90% of cervical cancers could be available in 2015.
Adding the TKI cediranib to chemotherapy improved progression-free survival in patients with metastatic or relapsed cervical cancer, according to a study presented at the 2014 ESMO Congress.

The US Food and Drug Administration has approved bevacizumab (Avastin) for the treatment of patients with recurrent or metastatic cervical cancer.
Researchers found that uterine cancers were present in 27 per 10,000 women undergoing morcellation, which fragments the uterus and can spread cancer cells.

In light of the recent FDA approval of HPV testing for women as a screening method for cervical cancer, we discuss changing guidelines with two experts.
A new clinical practice guideline from the American College of Physicians recommends against conducting routine pelvic exams in average-risk, asymptomatic women.

A new guide developed by nurses and patients with gynecologic cancer offers a much-needed practical resource for women struggling to understand the impact of cancer treatment on their sexual health.



The University of North Carolina has multiple posters accepted to the Gynecologic Oncology General Poster Session at this year’s ASCO meeting. Let’s take a virtual walk through several of these abstracts.

In this interview we discuss the diagnosis and treatment of endometrial cancer, a gynecologic cancer that forms in the tissue lining the uterus.

The FDA has approved an HPV DNA test to be used as a primary screening method for cervical cancer in women 25 and older. The test can also give insight into future risk of cervical cancer.

An FDA panel has recommended that a DNA test that screens for HPV in women can replace the standard Pap smear as a first-line primary cervical cancer screening test.

The Society of Gynecologic Oncology (SGO) recently issued two new clinical practice statements recommending genetic testing for all women with endometrial and ovarian cancers, regardless of family history.
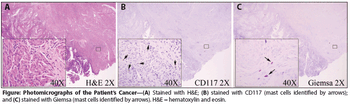
A 71-year-old woman not on hormone replacement therapy presented with uterine bleeding. Dilation and curettage revealed complex hyperplasia with atypia, focal clear-cell features, and endocervicitis. Endometrial intraepithelial carcinoma was suspected.
A new study found that high concentrations of vitamin C increased the effectiveness of chemotherapy in an ovarian cancer mouse model. A high-dose delivery of the vitamin also resulted in lesser toxicity from chemotherapy in cancer patients.
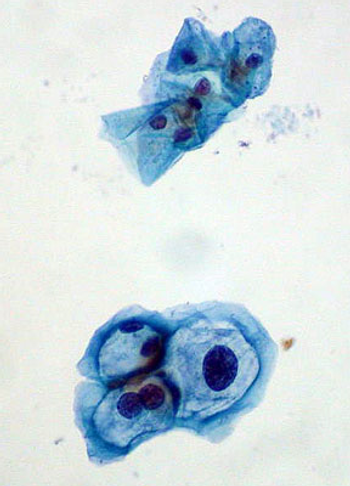
A new study has demonstrated that a therapeutic vaccine against HPV can stimulate an immune response and regression of high-grade cervical dysplasia, a precursor to cervical cancer in women with an HPV infection.


This article represents the consensus opinion of an expert panel and may be used to inform clinical recommendations in vaginal cancer management.

An analysis of four randomized clinical trials from Europe shows that HPV-based screening resulted in a greater long-term protection from invasive cervical cancer compared with a Pap test.

As advances in treatment strategies continue to focus on individualization of therapy, the identification of disease subsets is crucial to strategizing optimal therapeutic approaches.

In this review, the results and limitations of studies concerning adjuvant radiation therapy and chemotherapy for endometrial cancer will be discussed, focusing on evidence that can help to guide treatment decisions.

Future directions, including nomograms, multi-modality approaches, and more individualized patient care based on genomic profiles, may help to tailor each endometrial cancer patient’s therapy to her individual risk.

Taking advantage of the copious amounts of data generated from The Cancer Genome Atlas (TCGA), researchers have created a comprehensive map of the viruses present in a wide range of cancer types.

Researchers have demonstrated that a two-step screening test can identify ovarian cancer early, before the disease progresses to an advanced, poor prognosis stage.