
The U.S. Food and Drug Administration (FDA) announced last week the approval of brentuximab vedotin, a CD30-directed antibody drug-conjugate, for the treatment of refractory Hodgkin lymphoma and systemic anaplastic large-cell lymphoma.

Your AI-Trained Oncology Knowledge Connection!


The U.S. Food and Drug Administration (FDA) announced last week the approval of brentuximab vedotin, a CD30-directed antibody drug-conjugate, for the treatment of refractory Hodgkin lymphoma and systemic anaplastic large-cell lymphoma.

Equine ATG has been used for the treatment of severe aplastic anemia since the 1980s. Rabbit ATG is used in many parts of the world including South America, Japan, and European countries. The results of a randomized study of equine versus rabbit ATG showed that rabbit ATG was inferior to equine ATG.

Researchers at the University of Pennsylvania have reported on the results of a trial in which a patient with chronic lymphocytic leukemia (CLL) experienced a complete remission after immunotherapy with tumor-reactive modified T cells.
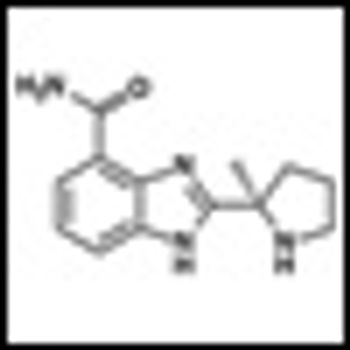
Results of a Phase I study of the novel Poly(ADP-ribose) polymerase (PARP) inhibitor veliparib (ABT-888) in solid tumors and lymphoma have just been published in Cancer Research (doi:10.1158/0008-5472.CAN-11-1227).
Researchers at the University of Chicago and colleagues have identified two variants on chromosome 6q21 that are associated with second malignant neoplasms (SMNs) in survivors of pediatric Hodgkin’s lymphoma. The SMNs are linked to radiation therapy used to treat the pediatric cancer.

Scientists at the University of British Columbia have found that histone-modifying genes are often mutated in non-Hodgkin’s lymphoma (NHL).

There has been dramatic progress in the management of acute promyelocytic leukemia during the past three decades. Important insights into the pathogenesis of the disease have come to light and effective treatment has been developed.

Our ability to stratify patients with CLL into high-risk and low-risk categories has advanced dramatically over the past two decades. However, which test or tests are most reliable remains to be seen.

The last decade has seen major changes in the treatment of chronic lymphocytic leukemia (CLL), with randomized trials now demonstrating improved survival with the use of chemoimmunotherapy.[1]

In their scholarly article, Dr. Park and Dr. Tallman review the important clinical trials for treating patients with APL reported over the last two decades and argue the case for further reduction and perhaps elimination of conventional cytotoxic chemotherapy in the frontline treatment of this disease.[1]

In this excellent review of acute promyelocytic leukemia (APL) treatment, the authors highlight opportunities offered by incorporating arsenic trioxide (ATO) into the therapeutic armamentarium.

To find a new cure for a disease, or at least to significantly prolong patient survival, requires a bit of insight and a lot of luck.

The current dogma considers cytomegalovirus (CMV) seropositivity being associated with inferior outcomes post hematopoietic stem cell transplant (HSCT). However, there has been a notion of “virus-versus-leukemia” effect since the 1980s; and recently, there have been some interesting reports which may turn this to a hot topic.

A rarely noted aspect of the era of novel agents and explosive new knowledge in the clonal plasma cell diseases is how short the half-life of relevant information has become, and how this churning has challenged clinical thinking.

In this issue of ONCOLOGY, Dr. Robert Kyle and colleagues provide their clinical and epidemiological perspective on monoclonal gammopathy of undetermined significance (MGUS) and smoldering myeloma (SMM)-a perspective that is based on more than three decades of experience.

In his plenary address as outgoing president of ASCO, Dr. George Sledge proposed that we are on the brink of a new era in cancer therapy – an era of genome-based treatment. He stressed that this new “genomic era” holds great promise for patients, citing as evidence a recent paper in JAMA that described a case in which the results of deep sequencing of a patient’s leukemic cells led to successful individualized therapy.
A growing understanding of the biology behind myelodysplastic syndromes (MDS) is leading the way to improved treatment options, according to two presentations at the American Society of Clinical Oncology (ASCO) Annual Meeting in Chicago.
Despite dramatic improvements in survival for children and young adults with acute lymphoblastic leukemia (ALL), treatment failure in the central nervous system remains a constant foe.

ONCOLOGY talks with Dr. Susan O’Brien, professor in the department of leukemia at the MD Anderson Cancer Center. Dr. O’Brien will be one of the presenters at the upcoming ASCO session on therapies for chronic lymphocytic leukemia, and she gives us a preview of what some of the highlights of the session are likely to be, as well as some insights into her own work.
In an article published online on May 9, 2011 in the Journal of Clinical Oncology, a large-scale prospective study found that acetaminophen use was associated with an almost two-fold increase risk of hematological malignancies other than chronic lymphocytic leukemia/small lymphocytic lymphoma.

Myelodysplastic syndromes, also referred to collectively as MDS, have significant biological and clinical heterogeneity, a highly variable natural history, and a complex pathobiology that is not clearly understood.

The myelodysplastic syndromes (MDS) are a heterogeneous spectrum of clonal hematopoietic diseases characterized by bone marrow hypercellularity, dysplasia of cellular elements, and consequent inadequate hematopoiesis, with resultant peripheral blood cytopenias.

The review by Dr. Akhtari outlines the diagnosis, prognosis, and treatment options for patients with myelodysplastic syndromes (MDS), and touches on the current challenges in treating patients suffering from MDS.

This review will cover the key elements of modern acute lymphoblastic leukemia treatment regimens, focusing primarily on front-line treatment and concluding with a brief discussion of the management of relapsed disease.

We have witnessed remarkable gains in the biological understanding and treatment of acute lymphoblastic leukemia (ALL) over the past decade.